Mechanistic Insights into Immune Synapse Formation During Immune Response
ibidi Blog | December 13, 2021 | Manuel Izquierdo, Universidad Autónoma de Madrid, Madrid, Spain
The cellular immune response exerted by T lymphocytes is essential in the body's fight against pathogens, including the virus responsible for COVID. The most effective vaccines are those capable of inducing an efficient and lasting immune response, both humoral (activation of B lymphocytes and production of antibodies) and cellular (activation of T lymphocytes and generation of memory T lymphocytes) in the immunized organism. Memory T cells "remember the pathogen" and have an antigen receptor capable of recognizing the pathogen's antigens (e.g., a virus) the next time an infection occurs to kill the infected cell. Knowing the molecular bases that regulate the activity of T lymphocytes specifically directed against pathogens, in the context of the immune synapse formation, is key to favoring and modifying lymphocyte responses, and will contribute to the development of new vaccination strategies.
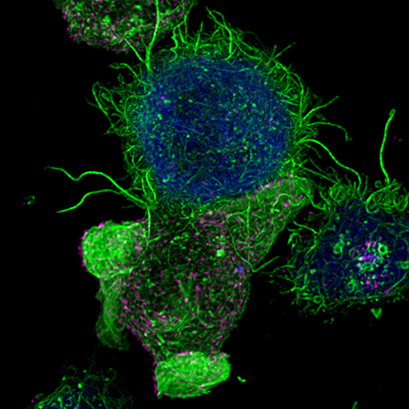
| Confocal image of a Jurkat T lymphocyte (bottom) forming an immunological synapse with an antigen-presenting cell (top, blue). Filamentous actin is visualized in green. Multivesicular bodies in magenta. Cells were imaged on an ibidi µ-Slide 8 Well . |
T and B lymphocyte activation by antigen-presenting cells (APC) occurs at a specialized cell-to-cell interface called the immunological synapse (IS). IS establishment by T and B lymphocytes is a very dynamic, plastic, and critical event, acting as a tunable signaling platform that integrates spatial, mechanical, and biochemical signals which are involved in antigen-specific, cellular and, humoral immune responses (de la Roche et al., 2016) (Fooksman et al., 2010). Thus, IS formation by T and B lymphocytes constitutes a crucial event during immune responses.
IS formation is associated with an initial increase in cortical F-actin at the IS (Billadeau et al., 2007), followed by a decrease in F-actin density at the central region of the IS that includes the secretory domain (Griffiths et al., 2010; Ritter et al., 2010). The underlying actin-regulating signaling pathways include phosphorylation processes by protein kinase C (PKC) family members (Griffiths et al., 2010; Xie et al., 2013)
After IS formation by T lymphocytes and antigen-presenting cells (APC), the convergence of secretory vesicles, including multivesicular bodies (MVBs) towards the microtubule organization center (MTOC), and MTOC polarization to the IS, are involved in polarized secretion at the synaptic cleft. The polarized secretion of exosomes at the immunological synapse is a developing and challenging area of research involved in relevant immune responses (Colombo et al., 2014). It has been demonstrated that multivesicular bodies (MVB), which are intracellular granules decorated with CD63 carrying intraluminal vesicles (ILVs), experience polarized transportation towards the IS (Alonso et al., 2011; Mazzeo et al., 2016; Video 1) upon TCR stimulation with antigen. The fusion of these MVB at the synaptic membrane induces their degranulation and the release of the ILVs as exosomes to the synaptic cleft (Alonso et al., 2011; Mittelbrunn et al., 2011).
|
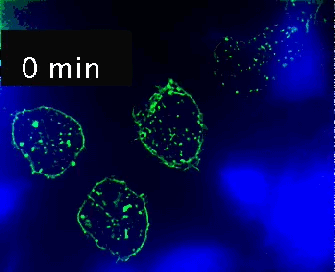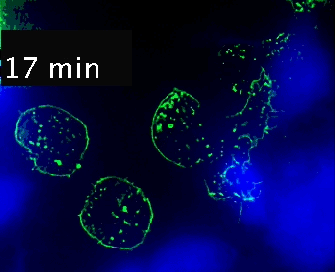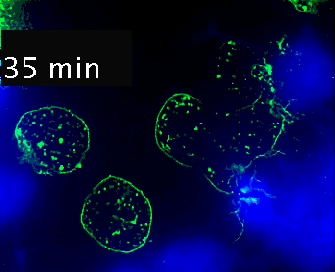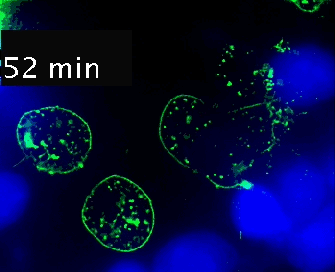
Live cell imaging (52min; 3 fps) captures the formation of a double synapse between a GFP-CdC63 transfected Jurkat cell (green) and two Raji cells (blue) and the subsequent polarized traffic of multivesicular bodies (GFP-CD63-decorated vesicles) to the synaptic contact areas in the cell forming synapses (center), but not in the two Jurkat cells that do not form synapses (left). Cells were imaged on the ibidi µ-Slide 8 Well. |
This specialized mechanism appears to specifically provide the immune system with a finely-tuned strategy to increase the efficiency of crucial secretory effector functions of T lymphocytes while minimizing nonspecific, cytokine-mediated stimulation of bystander cells, target cell killing, and activation-induced cell death (Calvo and Izquierdo, 2018).
The molecular bases involved in the polarized secretory traffic towards the IS in T lymphocytes have been the focus of interest; thus different models and several imaging strategies have been developed in order to gain insights into the mechanisms governing directional secretory traffic.
Several state-of-the-art, live cell-based imaging approaches have been developed to address the molecular mechanisms underlying this crucial immune secretory response, as summarized in (Calvo and Izquierdo, 2018).
Our lab developed a protocol that allowed us to visualize the early stages of IS formation and the subsequent polarized secretory traffic towards the IS employing live-cell imaging (Video 1).
Therefore, we have challenged Raji B cells coated with Staphylococcal enterotoxin E (SEE) superantigen (Montoya et al., 2002) with Jurkat T cells. This can be done in cultured cells in suspension but also using Raji B cells attached to fibronectin-coated coverslips ( ibidi µ-Slide 8 Well ).
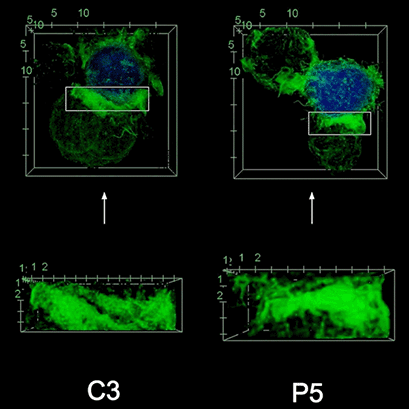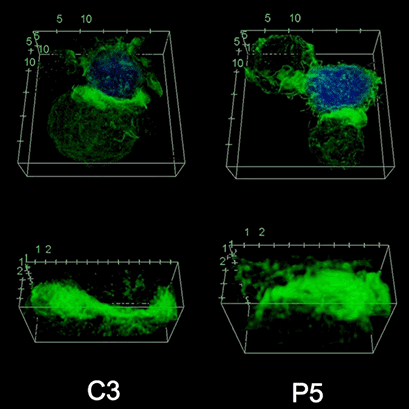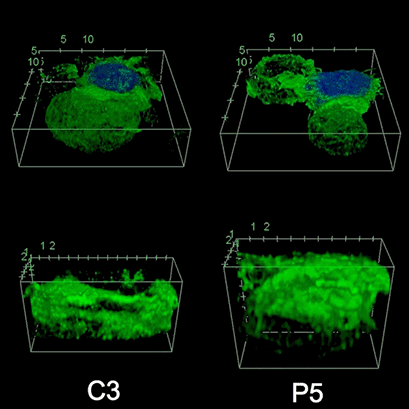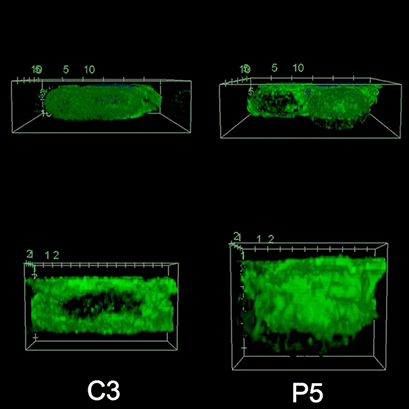
To investigate the role of F-actin reorganization at the synaptic area during synapse formation, we compared the actin-architecture in control and PKCdelta-interfered human Jurkat clones. Subsequent fixation and immunolabelling revealed a depletion of F-actin at the central region of the synaptic interface in the control, but not the P5 PKCdelta-interfered clone (Video 2). This result leads to the conclusion that PKCdelta dependent removal of F-actin is required for efficient exosome secretion.
|
3D Z-Projection of fixed Jurat cells (control, C3 and PKCdelta, P5), labeled with CMAC (blue) 1h after synapse conjugate formation. F-actin was visualized with phalloidin-488 (green). The white rectangles in frame no. 1 correspond to the immunological synapse interface. First photogram of the video corresponds to a top view of the synaptic conjugate, whereas the last frame shows the immunological synapse contact area (synaptic interface) at a 90-degree angle to the observer. Upper panels: merged CMAC and phalloidin channels. Lower panels: enlarged immunological synapse area (1.5x and 2.5x zoom for control cell (C3) and and PKCdelta cell (P5); white rectangles in the upper panels) of phalloidin channel. The accumulation of F-actin at the synaptic contact areas (enclosed in white rectangles) is evident in both cells. However, the depletion of F-actin at the central region of the synaptic interface is observed in the control, but not in the P5 PKCdelta-interfered, which was visualized when the synaptic interface is at a 90-degree angle to the observer at the end of the video. Confocal imaging of cells cultured on the ibidi µ-Slide 8 Well. |
To find out how to use the versatile ibidi µ-Slide 8 Well for live cell imaging and subsequent immunofluorescence stainings, watch this JOVE video for a detailed protocol: https://www.jove.com/de/t/60312/imaging-the-human-immunological-synapse
or download the ibidi UP09 " Protocol for Live Cell Imaging of the Immune Synapse" .
Manuel Izquierdo, PhD Manuel obtained his PhD at the Hospital de la Princesa (Madrid) with a thesis on immune reconstitution of T cells after bone marrow transplantation (1986-1990). Manuel then worked as a postdoctoral fellow (1990-1994) at the ICRF (London, UK) on the intracellular signals involved in the activation of p21ras and the function of p21ras in T lymphocytes. He then returned to Spain and worked as a postdoctoral fellow (1994-1996) at the Institute of Biomedicine and Parasitology "López Neyra" (CSIC, Granada), where he studied the signals involved in apoptosis of T lymphocytes. Subsequently (1997-1999), Manuel worked as a postdoctoral fellow in the Department of Immunology and Oncology of the CNB (CSIC, Madrid), studying the function of caspases in negative selection processes in the thymus. In 2000, Manuel obtained a Tenured Scientist position at the CSIC (IBGM, CSIC, Valladolid), where he worked as Principal Investigator in two different areas: 1) study of the intracellular signals involved in the activation and programmed cell death of T lymphocytes; 2) study of the molecular mechanisms involved in the secretion of exosomes at the immunological synapse made by T lymphocytes. In 2004, Manuel moved to IIB "Alberto Sols" (IIBM, CSIC, Madrid), where he was promoted to Senior Researcher (CSIC) in 2009 and remains active to date. Manuel is interested in the application of advanced image techniques in living T lymphocytes (High Resolution Image capture and analysis) as well as multidisciplinary approaches (Nanotechnology) to the study of the immunological synapse. |
|
REFERENCES
- Alonso, R., Mazzeo, C., Rodriguez, M.C., Marsh, M., Fraile-Ramos, A., Calvo, V., Avila-Flores, A., Merida, I., and Izquierdo, M. (2011). Diacylglycerol kinase alpha regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ 18 , 1161-1173.
- Billadeau, D.D., Nolz, J.C., and Gomez, T.S. (2007). Regulation of T-cell activation by the cytoskeleton. Nat Rev Immunol 7 , 131-143.
- Calvo, V., and Izquierdo, M. (2018). Imaging Polarized Secretory Traffic at the Immune Synapse in Living T Lymphocytes. Front Immunol 9 , 684.
- Calvo, V., and Izquierdo, M. (2021). Role of Actin Cytoskeleton Reorganization in
- Polarized Secretory Traffic at the Immunological Synapse. Front. Cell Dev. Biol., 4
- Colombo, M., Raposo, G., and Théry, C. (2014). Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annual Review of Cell and Developmental Biology 30 , 255-289.
- De La Roche, M., Asano, Y., and Griffiths, G.M. (2016). Origins of the cytolytic synapse. Nat Rev Immunol 16 , 421-432.
- Fooksman, D.R., Vardhana, S., Vasiliver-Shamis, G., Liese, J., Blair, D.A., Waite, J., Sacristan, C., Victora, G.D., Zanin-Zhorov, A., and Dustin, M.L. (2010). Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol 28 , 79-105.
- Fuss, I.J., Kanof, M.E., Smith, P.D., and Zola, H. (2009). Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr Protoc Immunol Chapter 7 , Unit7 1.
- Griffiths, G.M., Tsun, A., and Stinchcombe, J.C. (2010). The immunological synapse: a focal point for endocytosis and exocytosis. J Cell Biol 189 , 399-406.
- Jambrina, E., Alonso, R., Alcalde, M., Del Carmen Rodriguez, M., Serrano, A., Martinez, A.C., Garcia-Sancho, J., and Izquierdo, M. (2003). Calcium influx through receptor-operated channel induces mitochondria-triggered paraptotic cell death. J Biol Chem 278 , 14134-14145.
- Mazzeo, C., Calvo, V., Alonso, R., Merida, I., and Izquierdo, M. (2016). Protein kinase D1/2 is involved in the maturation of multivesicular bodies and secretion of exosomes in T and B lymphocytes. Cell Death Differ 23 , 99-109.
- Mittelbrunn, M., Gutierrez-Vazquez, C., Villarroya-Beltri, C., Gonzalez, S., Sanchez-Cabo, F., Gonzalez, M.A., Bernad, A., and Sanchez-Madrid, F. (2011). Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2 , 282.
- Montoya, M.C., Sancho, D., Bonello, G., Collette, Y., Langlet, C., He, H.T., Aparicio, P., Alcover, A., Olive, D., and Sanchez-Madrid, F. (2002). Role of ICAM-3 in the initial interaction of T lymphocytes and APCs. Nat Immunol 3 , 159-168.
- Ritter, A.T., Asano, Y., Stinchcombe, J.C., Dieckmann, N.M., Chen, B.C., Gawden-Bone, C., Van Engelenburg, S., Legant, W., Gao, L., Davidson, M.W., Betzig, E., Lippincott-Schwartz, J., and Griffiths, G.M. (2015). Actin depletion initiates events leading to granule secretion at the immunological synapse. Immunity 42 , 864-876.
- Xie, J., Tato, C.M., and Davis,M.M. (2013). How the immune system talks to itself: the varied role of synapses. Immunol. Rev. 251, 65–79. doi: 10.1111/imr.12017
 (1)
(1)  (1)
(1)