3D Cell Culture Models – An Overview
ibidi Blog | December 20, 2022 | Tina Freisinger, ibidi GmbH
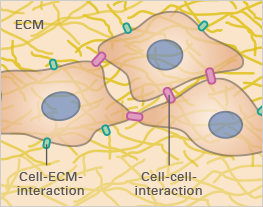
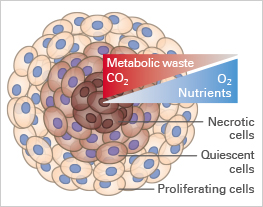
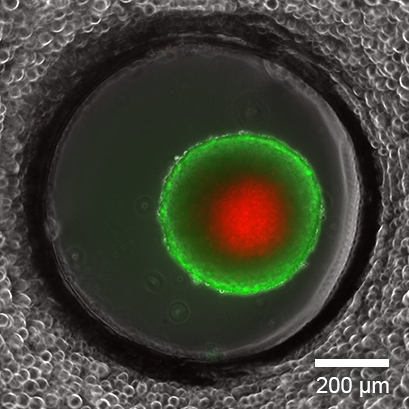
In vitro cell culture is an essential tool for studying cellular function, understanding disease, and discovering new drugs. The majority of these experiments are being carried out with cells cultivated as two-dimensional monolayers on tissue culture-treated plastic vessels. However, the in vivo environment of living cells looks completely different. Cells are embedded in tissue composed of other cells and structural components, called the extracellular matrix (ECM; Fig. 1A). The cell-to-cell and cell-to-ECM interactions and the mechanical forces within these tissues have a crucial impact on cell morphology and behavior. Moreover, the exposure to nutrients, metabolites, oxygen, and CO2 in living tissue or 3D cell structures (e.g., tumors) is very different from 2D monolayers (Fig. 1B and 1C).
|
|
| ||
Fig. 1A | Fig. 1B | Fig. 1C |
Researchers must consider these factors before establishing a cell culture model to investigate cellular function, a specific disease (e.g., cancer), or characterize certain drugs. In order to receive the most in vivo -like experimental results, it also makes sense to mimic the physiological conditions of living tissue as closely as possible.
In this article, we introduce different 3D cell culture models to study cells in an in the vivo-like state.
From Cell Monolayers to Tissues
While cells are still predominantly cultivated in 2D monolayers on cell culture-treated plastic surfaces or coated with proteins or a thick cell matrix (2.5D culture), you can choose from various 3D cell culture models when you are interested in cultivating cells in a more physiological way (Fig. 2).
It is possible to use scaffold-based techniques such as polymers or hydrogels, which provide structural support through synthetic chemical polymers or a network of proteins. Alternatively, a three-dimensional cell structure can be obtained using scaffold-free techniques, where complex cell aggregates or whole tissues can form without additional structural support.
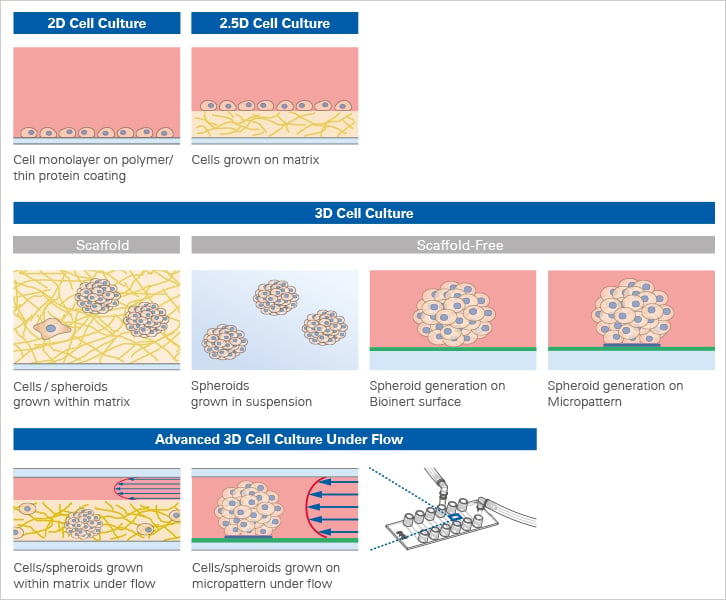
Fig. 2: Overview of 2D and 3D Cell Culture Models.
Both approaches have pros and cons that should be considered when deciding which technique is the most appropriate for the respective research question. Scaffold methods take cell-to-ECM interactions into account, whereas the environment of the cells can be better manipulated with spheroids grown in suspension.
For long-term cultivation and investigation of 3D structures on the microscope, special tools and techniques have been developed to ensure a constant media supply and simulation of physiological conditions over an extended period of time.
Scaffold-Based 3D Cell Culture Models
Scaffolds for 3D cell culture models can range from porous polymeric membranes to complex hydrogels that mimic the cellular microenvironment of the ECM.
In general, hydrogels form a structure of crosslinked polymers, which can absorb and bind large amounts of water. At 4°C or room temperature, the mixture is liquid, but at 37°C it forms a gel. This feature makes it possible to mix the liquid with cells, which are then embedded in a three-dimensional matrix when incubated at 37°. This allows the cells to retain specific in vivo features such as cell-ECM interaction or physiological and mechanical properties of the environment.
There are different types of hydrogels, for example, Matrigel® or collagen, which are derived from organic sources. Then, there are complex synthetic hydrogels with the advantage of having precise control over their composition (molecules, crosslinkers, etc.). This allows for drawing distinct conclusions on the effect of the individual components.
Find out more about Matrices for 3D Culture.
Scaffold-Free 3D Cell Culture Models
Scaffold-independent methods allow for the cultivation of cells in 3D without the support of a matrix. They rely on the self-assembly probabilities of cells into aggregates or spheroids. A variety of techniques are established to facilitate this self-assembly process. Hanging Drop TechnologyThe hanging drop technology is a well-established method in biology and has been used for many different applications, such as the observation of whole (micro)organisms or the crystallization of proteins. It takes advantage of gravity forces that lead to the formation of a suspended liquid droplet hanging down from a glass plate. Over the past decade, this technique has been modified and adapted for 3D cell culture applications, allowing spheroids to form from a cell suspension without the requirement of a supporting scaffold (Fig. 4). Today, specialized hanging drop plates are commercially available. However, while this method is suitable for the generation of spheroids, it is not possible to combine it with high-resolution microscopy. |
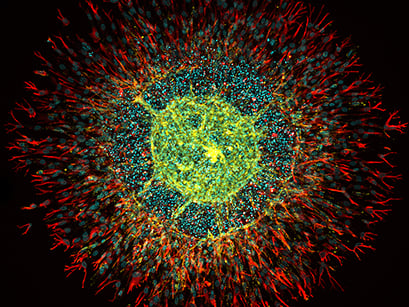
Fig.3: Spheroid of human intestinal fibroblasts and endothelial cells sprouting in a fibrin hydrogel cultured in a µ-Slide 4 Well. Endothelial cells were stained with CD31 (yellow), fibroblasts with vimentin (red), and DNA with DAPI (cyan). Confocal image by H. Nogueira Pinto, Bioengineered 3D Microenvironments Group, Instituto de Investigação e Inovação em Saúde (i3S), Universidade do Porto, Portugal. |
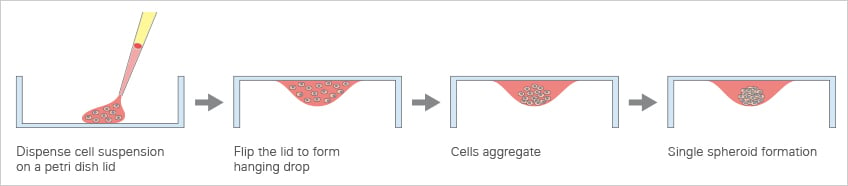
Fig. 4: Principle of the hanging drop method.
Ultra-Low Attachment (ULA) Coating
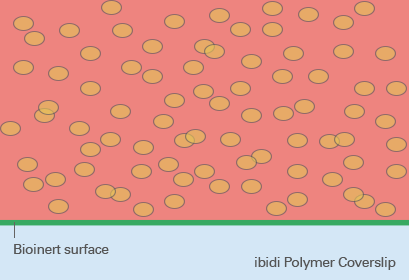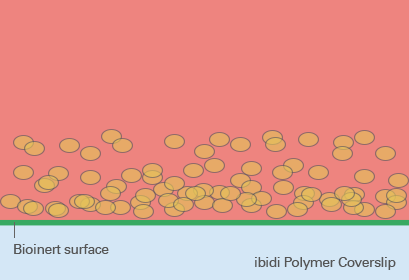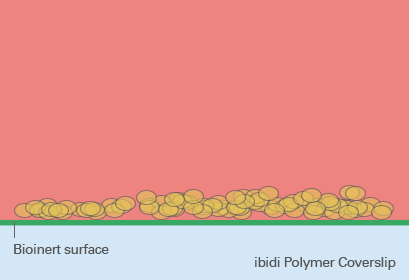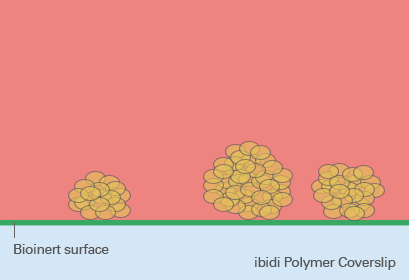
So-called low adhesion plates are coated with neutrally charged or hydrophilic proteins or hydrogels that reduce the attachment of cells to the plate surface and therefore enable the formation of 3D cell structures. Although this coating predominantly prevents the attachment of cells, cell adhesion can still occur after a more extended period of time because the coating is often just physisorbed and not covalently bound – and therefore not long-term stable. Bioinert SurfacesIn contrast to standard ULA coatings, a Bioinert surface is covalently bound and provides stable surface passivation. Therefore, it prevents protein or cell attachment to the coated surface completely. This promotes cell-to-cell interaction and 3D cell aggregate formation, even in long-term experiments (Fig. 5). |
Fig. 5: Spheroid formation on Bioinert surface on ibidi Polymer Coverslip. |
Find out more about ibidi Bioinert surfaces.
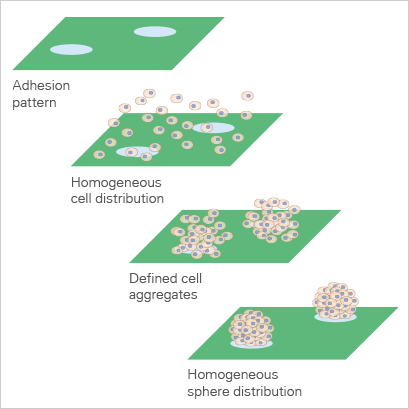
Micropatterned Surface Microplates
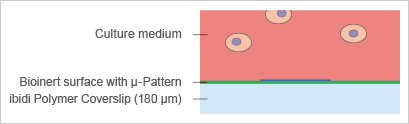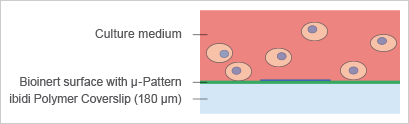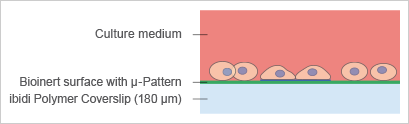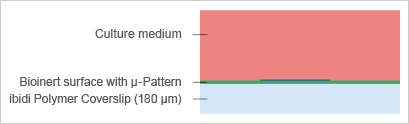
The imprinting of micropatterns on Bioinert surfaces allows for the precise adhesion of cells in defined locations (Fig. 6). The passivated area surrounding the µ-patterned spots facilitates the formation of 3D cell aggregates and spheroids (Fig. 7 and 8). |
Fig. 6: Animation of cell attachment on micropatterned spots. |
Fig. 7: Principle of spheroid formation on micropatterned spots. |
Fig. 8: Suspension of NIH-3T3 cell line seeded on 200 µm adhesion spots, 64 hours live cell imaging, phase contrast, 4x objective lens. |
Find out more about the ibidi µ-Pattern Technology.
Microplates with Specialized Geometries, Organ-On-A-Chip Microfluidics, and Devices for Long-Term 3D Cell Cultivation
In an attempt to bring in vitro cell culture as close as possible to in vivo conditions, the fabrication of organ-on-a-chip assays in combination with perfusion devices has become increasingly important. For this application, cells are embedded in matrices which are placed into microchannels. These channels can be perfused with media or drugs for long-term cell cultivation under flow or drug screening (Fig. 9 and 10).
Microplates with specialized geometries allow specific 3D cell-based assays (e.g., wound healing or chemotaxis assays) to be conducted or mimic different organs (e.g., lung or liver).
|
| |
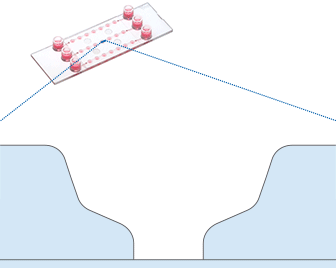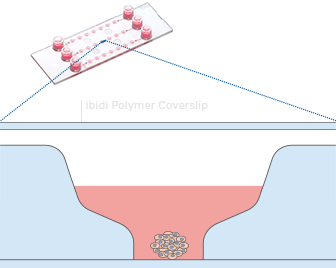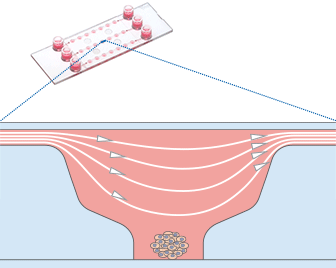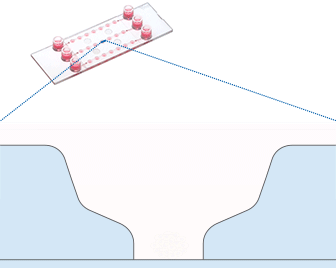
Fig 9: Working principle of the ibidi µ-Slide Spheroid Perfusion, which can be connected to a pump system (e.g., the ibidi Pump System) for long-term cultivation of spheroids. | Fig. 10: L929 fibroblasts show spheroid formation in the µ-Slide Spheroid Perfusion, Bioinert, days 1–14, seeding concentration 5 x 105 single cells/ml. Left: no perfusion, medium exchange every second day. Right: perfusion with the ibidi Pump System, 0.75 ml/min. Phase contrast microscopy, 10x objective lens, well diameter 800 µm. |
Find out more about Cell Culture Under Flow.
In summary, huge progress has been made to mimic the physiological conditions of living tissue by moving from 2D cell monolayers to 3D cell cultures. This allows for better readouts of cell-based assays, disease models, and drug effects, hence making cell culture in general more accurate and a better alternative to animal models.
For more information on spheroids, organoids, and 3D Cell Culture, visit our 3D Cell Culture application chapter.
References:
Kim W, Gwon Y, Park S, Kim H, Kim J. Therapeutic strategies of three-dimensional stem cell spheroids and organoids for tissue repair and regeneration. Bioact Mater. 2022 Apr 4;19:50-74. doi: 10.1016/j.bioactmat.2022.03.039.
Read article
Krysko DV, Demuynck R, Efimova I, Naessens F, Krysko O, Catanzaro E. In Vitro Veritas: From 2D Cultures to Organ-on-a-Chip Models to Study Immunogenic Cell Death in the Tumor Microenvironment. Cells. 2022 Nov 21;11(22):3705. doi: 10.3390/cells11223705.
Read article
Langhans SA. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front Pharmacol. 2018 Jan 23;9:6. doi: 10.3389/fphar.2018.00006.
Read article
Clevers H. Modeling Development and Disease with Organoids. Cell. 2016 Jun 16;165(7):1586-1597. doi: 10.1016/j.cell.2016.05.082.
Read article
 (7)
(7)  (0)
(0)