Immunofluorescence Staining: A Typical Workflow
Every immunofluorescence staining protocol consists of four major steps (cultivation, fixation, staining, imaging), which can be subdivided as follows:
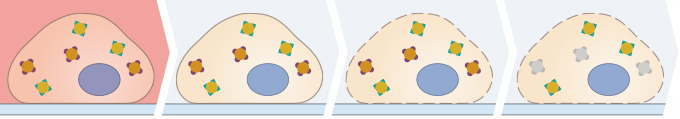 | ||||
| Experiment Planning and Sample Preparation | Sample Fixation | Cell Permeabilization | Blocking | |
| Primary Antibody Incubation | Secondary Antibody Incubation | Counterstain and Mounting | Microscopy | |
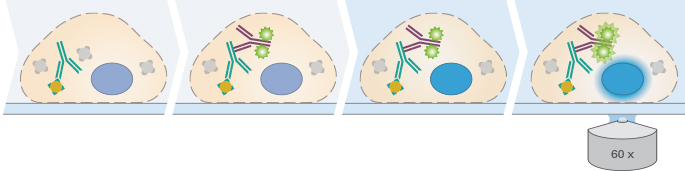 | ||||
Immunofluorescence staining is a very sensitive method that might require troubleshooting. Slight changes in the protocol can lead to different results that are no longer comparable. Therefore, it is very important to precisely maintain the exact same conditions in your specific protocol (e.g., cell density, antibody dilution, incubation temperature, and incubation time). The following is an overview of the different steps of an indirect immunofluorescence staining protocol.
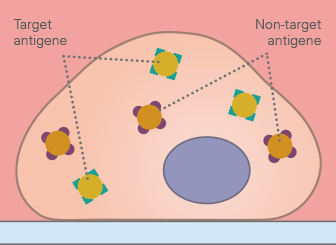

Before starting an immunostaining, a literature research should be done to determine the expression levels and intracellular localization of the protein of interest in the chosen model system. Some proteins have a very low expression, either generally, or in certain cell lines. In this case, the expression might have to be induced by external stimuli or overexpression techniques.
Further, the optimal cell density has to be determined. In general, a confluence of 70%–80% is recommended for immunocytochemistry.
In addition, the ideal cell culture vessel geometry and substrate/coating must be determined, and needs to be compatible with the chosen microscopy method. During the whole experiment, it is crucial that the cells never dry out, which should be considered when choosing the appropriate cell culture vessel geometry.
Finally, the number of samples to be stained and analyzed for statistical significance, including the appropriate controls, should be planned in advance. For complex approaches, creating a schematic of the experimental conditions, including the planned stainings—with both positive and negative controls—is recommended.
Continue reading about the compatibility of different surfaces and microscopy techniques.
ibidi Solutions

The ibidi μ-Slides and µ-Dishes with a coverslip bottom for inverted microscopy are available in a variety of geometries that will fit any of your specific needs for immunocytochemistry. All immunofluorescence staining steps can be performed directly in the slides or dishes.
The geometry of the ibidi Channel Slides is ideal for the exact exchange of small medium amounts, which is necessary during immunocytochemistry stainings. In addition, the coverslip bottom of the channel µ-Slides eliminates the need for additional coverslips.
In the ibidi Chamber Slides, a silicone gasket with separated chambers is mounted on a standard glass slide. The slides are ideal for the long-term storage of samples that are mounted with a glass coverslip.
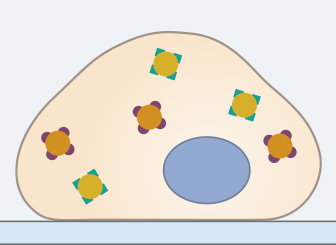

The first step of an immunofluorescence staining protocol is to fixate the sample. This is usually done by incubating the sample for 10 minutes at room temperature in a 4% formalin solution (in PBS, pH 7.4), which crosslinks the proteins. The sample can also be fixated in 100% chilled methanol or acetone.
Optimal fixation conditions should be determined individually for each experiment. Some proteins are destroyed by methanol fixation, and some antibodies do not detect proteins in formalin-fixated samples. After fixation, it is very important to wash the sample 3x for 5 minutes in a washing solution, (e.g., PBS) to remove the fixation solution completely.
ibidi Solutions

Unlike most plastic materials used for cell culture, the ibidi μ-Slides, µ-Dishes, and the ibidi Chamber Slides, removable, are compatible with standard fixation methods.
For a full overview, please check out our chemical compatibility table. All immunofluorescence staining steps can be performed directly in the slides or dishes.
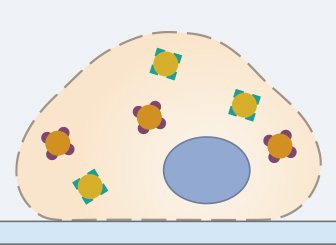

In order to stain intracellular proteins, the cell needs to be permeabilized. Without this step, it is not possible for the antibodies to enter the cell through the lipid membrane. The permeabilization requires incubation in a detergent, for example Triton X-100 or Tween-20 (for a less harsh permeabilization) in a PBS solution.
This step must be optimized depending on the protein of interest, the used antibody, and the experimental conditions. Especially when staining membranous proteins, the permeabilization step has to be done with caution, because Triton X-100 can destroy the cell membrane. In this case, using saponin might be an alternative to classic detergents. Don’t forget that methanol-fixed samples are already permeabilized, since alcohols easily wash out the lipids of the cell membrane.
After this step, the sample has to be washed 3x for 5 minutes in a washing solution.
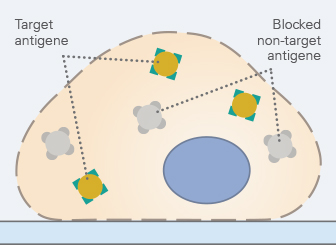

In order to minimize intra- or extracellular background signals, non-specific antigens should be blocked by incubating the sample in (1) the serum of the host, in which the secondary antibody was made, (2) bovine serum albumin (BSA), or (3) milk. The first option is the most recommended because of its highest specificity.
Typical blocking times are from 30 minutes up to one hour. Excessively long blocking steps should be avoided, as this can reduce the specific binding of the primary antibody, and therefore reduce the signal.
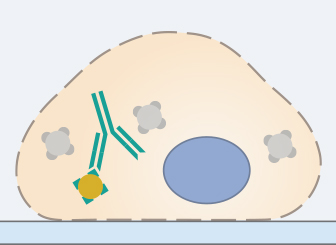

The selection of the primary antibody and its incubation conditions is the most critical step of an immunofluorescence staining protocol. Especially, if the protocol has not yet been established in the lab, a literature research is crucial. A suitable primary antibody must have a high specificity for the antigen of interest. Furthermore, whether the antibody is mono- or polyclonal influences its specificity. Many antibody manufacturers list references on their product pages, in which the antibody was successfully used in an immunofluorescence staining.
Another highly relevant property of the primary antibody is its originating host, as it determines the secondary antibody. This is especially important when doing multicolor stainings, because different specific primary antibodies for the parallel detection of several antigens in the same sample must be produced in different hosts, in order to avoid cross-reactivity.
The optimal incubation conditions must be determined carefully for each experiment. Antibody concentrations that are too high and incubation times that are too long can result in an unspecific background signal. Conversely, concentrations that are too low and incubation times that are too short can lead to a very weak or missing signal.
After incubation with the primary antibody, the samples should be washed 3x for 5 minutes in a washing solution in order to avoid background fluorescence.
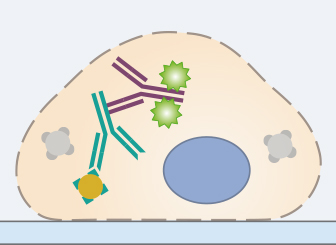

In standard immunofluorescence assays, the secondary antibody is conjugated to a fluorophore, which emits light when excited at a defined wavelength. The secondary antibody specifically binds to the first antibody. Therefore, it is essential that the secondary antibody is specific to the host in which the primary antibody was produced. For a successful experiment, the chosen microscopy technique and the equipment (filters, lasers, cameras, and detectors) also need to be compatible with the chosen fluorescence dye.
To be suitable for fluorescence microscopy, stable fluorophores must be used, because the sample is generally exposed to a high number of photons. Also, fluorophore brightness should be taken into account, because the antigen with the highest expression level should be detected with the fluorophore with the lowest brightness. When doing a multicolor staining, the spectral overlap of the conjugated fluorophores should be carefully considered as well. Respective tables can be found on the manufacturers’ websites.
The incubation in the secondary antibody solution should be carried out according to manufacturer’s protocol. Since fluorophores are sensitive to light, from now on all steps of the protocol need to be carried out in the dark.
In order to avoid background fluorescence, the samples should be washed 3x for 5 minutes in a washing solution after incubation with the secondary antibody.
Excurse: What is a fluorophore?
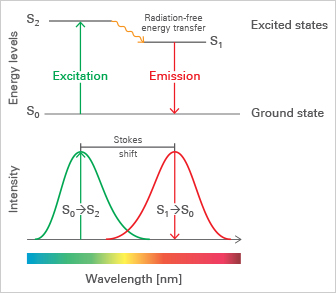
A fluorophore (also called fluorochrome) is a chemical molecule, which is used to label specific structures for microscopic analysis. For example, the green fluorescent protein (GFP), red fluorescent protein (RFP), and yellow fluorescent protein (YFP) are widely-used fluorophores in research.
Fluorophores are excited when they absorb light of a certain wavelength and then re-emit light at a longer wavelength through fluorescence. Light absorption excites the fluorophore from the ground state (S0) to an energy-rich excited state (S2 or S1). These excitation states are not stable and quickly undergo relaxation processes. First, excited fluorophores rapidly relax to the lowest energy level of S1 through non-radiating processes, known as internal conversion. This energy-consumptive process generally is complete prior to light re-emission, causing the re-emitted light to be of a lower energy and, therefore, of a longer wavelength than the absorbed light. This effect is known as Stokes-shift. Re-emission of Stokes-shifted light at a longer wavelength, known as fluorescence, finally relaxes the fluorophore back to its ground state S0.

The final step before microscopy is the counterstaining of the nuclei and the mounting. In order to avoid drying out of the samples, and to guarantee a stable refractive index of the cellular environment (a prerequisite for successful microscopy), the sample needs to be mounted.
For this, the sample should be covered with a mounting medium that has a low autofluorescence. DAPI, the standard for nuclear counterstaining, is either included in the mounting medium, or can be added separately.
ibidi Solutions

The ibidi Mounting Medium and the ibidi Mounting Medium With DAPI have a very low autofluorescence, prevent photobleaching and allow the sample to be stored for several weeks on the µ-Slide without the need for additional coverslips.
If your experiment requires long-term storage of immunostained samples, we recommend using the ibidi Chamber Slides, removable. Since signals could fade if fluorophores are exposed to light for a longer period, the samples always must be stored in the dark.
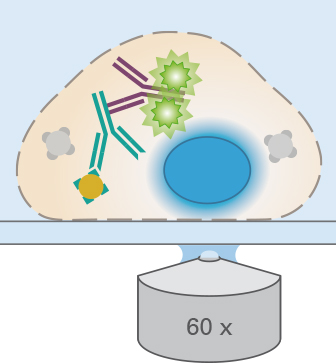
To get optimal results, the microscopic analysis of the immunofluorescence staining should be done directly after the mounting.
Many microscopy techniques exist, each optimized for different experimental approaches. For standard immunofluorescence stainings, epifluorescence and confocal microscopy are widely-used methods. Of course, all parameters such as magnification and exposure should be carefully and individually determined.
Explore a detailed overview of the different microscopy techniques and their applications here.
Read on and learn more about Immunofluorescence Troubleshooting, or about how to do Immunofluorescence Stainings With the ibidi Chambers.




