Cell Culture: General Cell Handling
Handling Cells in Culture
Starting at the end of the 19th century, scientists have been isolating cells out of their natural environments from various organisms (e.g., human, mouse, swine, chicken, and plants) and placing them in an in vitro (Latin “in the glass”) culture. The experimental cells have been extracted from a number of different tissues and organs, such as intestine, kidney, blood, brain, mammary, and many cancer entities.
The aim of culturing cells in vitro is to mimic the in vivo (Latin “in the living”) conditions inside an incubator or—when doing live cell imaging—even under a microscope. Every cell type requires tailored culture parameters for optimal health, viability, and growth. For all experiments, it is crucial to keep the conditions sterile, homogeneous and reproducible, in order to get comparable results.
Passaging, Seeding, and Freezing Cells
In order to keep cells in a proliferative state, and to prevent them from reaching overconfluence, it is important to passage them at defined intervals. Cell passaging must be done regularly, in order to keep the cells in culture, achieve volume upscaling, or to seed them at the start of an experiment. This procedure is also called cell splitting or subculturing. Among other factors, the optimal time for passaging depends on the proliferation rate, as well as the required cell number. The exact splitting protocol is unique for every cell type. Generally, adherent cells are detached from the substrate using proteolytic enzymes (mostly trypsin/EDTA), which are necessary for the digestion of their protein attachment bonds. Next, the cells are homogenized, counted, and seeded into a new vessel, thus increasing the passage number by one.
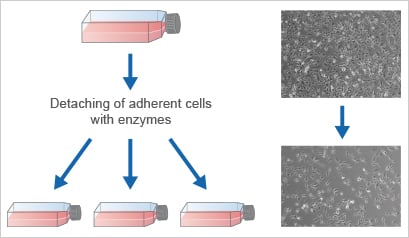
Passaging / subcultivation = transferring cells into fresh cultureware and growth medium
Some cell lines, such as HeLa or other cancer cells lacking cell cycle regulation, can be passaged infinitively without losing their ability to divide (immortal cell lines). Also, this is the case for some embryonic cell types or pluripotent stem cells. Other cells, especially primary cells (e.g., isolated cells from adult organs), will lose this ability after a few passages, because they either undergo senescence, they differentiate, or they die. Therefore, it is important to use cells with a similar passage number for reproducible experiments.
For cell seeding, choosing a density that is suitable for the respective cell type, cell culture vessel, and experimental setup is necessary. If the density is too low, the cells might stop growing due to an insufficient release of growth factors into the culture medium. Singular cells that lack cell-cell and cell-matrix communication might even die because of a special form of programmed cell death called anoikis. If the seeding density is too high, the cells might already reach overconfluence after a very short time. This leads to an impaired proliferation and cell detachment from the substrate, which results in non-reproducible experimental conditions.
Standard cell types can be stored for short-term periods at –80°C, or long-term in liquid nitrogen. To prepare the cells for this, they need to be trypsinized, homogenized, and centrifuged before freezing them using a special freezing medium. To counter the cell stress caused by this process, it is recommended to use a freezing media with high recovery rates. In addition, the thawing procedure must be carried out quickly (e.g., in a water bath at 37°C), before transferring the cells to the culture medium and putting them into an incubator to create physiological conditions.
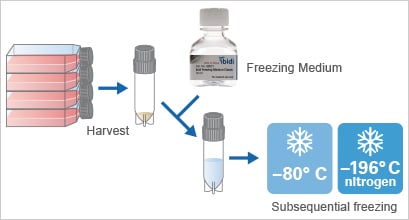
Cells can be stored for future use via freezing (= cryopreservation)
Further reading:
Gstraunthaler G., Lindl T., Zell- und Gewebekultur, Allgemeine Grundlagen und spezielle Anwendungen, 7. Auflage, Springer Spektrum, Berlin, Heidelberg, 2013, 10.1007/978-3-642-35997-2.
Freshney, R. I., Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications, 7th Edition, Wiley-Blackwell, Hoboken, 2016.
Read on to learn more about Parameters for Healthy Cells or Prevention of Contaminations.




