The Principle of Immunofluorescence Assays
Immunofluorescence (IF) is a powerful approach for getting insight into cellular structures and processes using microscopy. Specific proteins can be assessed for their expression and location, making immunofluorescence indispensable for scientists to solve many cell biological questions.
An immunofluorescence experiment is based on the following principal steps:
- Specific antibodies bind to the protein of interest.
- Fluorescent dyes are coupled to these immune complexes in order to visualize the protein of interest using microscopy.
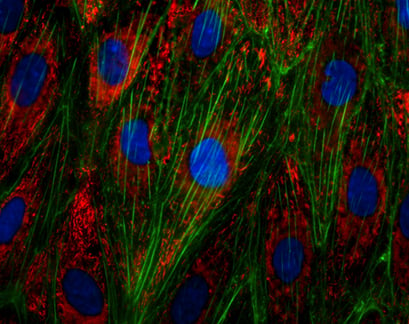
Immunofluorescence staining of the von-Willebrand-Factor (vWF) in endothelial cells (HUVECs). Actin was stained using phalloidin (green), nuclei are stained with DAPI (blue).
It is distinguished between direct and indirect immunofluorescence. In direct immunofluorescence, the primary antibody is directly coupled to a fluorophore (also called fluorochrome), allowing for easy handling and quick visualization. In indirect immunofluorescence, a secondary fluorophore-coupled antibody, which specifically binds to the primary antibody, is used to visualize the structure of interest.
Although the second approach is more time-consuming than direct immunofluorescence, it has several big advantages, such as it is generally less expensive, because the secondary antibody can be used for different primary antibodies. In addition, several proteins can be specifically visualized in parallel in one single sample (multicolor immunofluorescence) by combining multiple primary antibodies with specific secondary antibodies—each of them labeled with a different fluorophore.
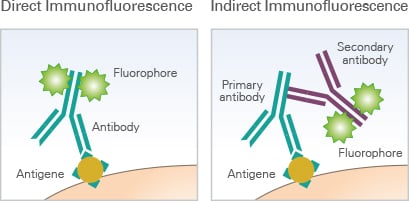
The principle of direct immunofluorescence and indirect immunofluorescence.
Read on and learn more about a typical Workflow of an Immunofluorescence Staining, or about how to do Immunofluorescence Stainings With the ibidi Chambers.




