Experimental Workflow of a Wound Healing Assay and Migration Assay
Before Starting
For a successful experiment, please address the following questions before starting.
Sample Preparation
Procedure
Here, the gap is created by using the ibidi Culture-Inserts, which guarantee extremely high reproducibility due to the defined size of the cell-free gap. Choose a suitable Culture-Insert in an appropriate cell culture vessel. Seed and culture the cells until they form an optically confluent monolayer. Let the cells grow for approximately 24 hours. If necessary, treat the cells with a proliferation inhibitor, then remove the Culture-Insert to create the gap.
ibidi Solutions

- The ibidi Culture-Insert 2 Well | 3 Well | 4 Well are silicone inserts with a defined, cell-free gap for wound healing, migration, 2D invasion assays, and co-cultivation of cells. They are available as individual inserts in a µ-Dish 35 mm or as 25 pieces in a transport dish for self-insertion, and provide a quick and easy experimental setup.
- The Culture-Insert 2 Well 24 is a ready-to-use solution in a 24 well plate for cost-effective high throughput assays.
- The ibidi µ-Slide 2 Well contains 2 wells that have the ideal size for the insertion of Culture-Inserts.
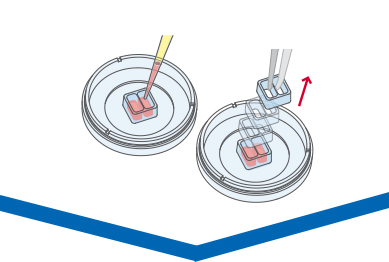
Live Cell Imaging
Procedure
The cells should be monitored immediately after the gap creation, in most cases by using phase contrast microscopy. It is possible to take pictures of the gap at different time points (e.g., after 6 and 12 hours) or do live cell imaging. Usually, it is sufficient to monitor the cells for 24 hours or less. For an accurate analysis, it is important to observe the exact same gap position during the entire experiment.
ibidi Solution

The ibidi Stage Top Incubation System provides a physiological environment under the microscope, enabling live cell imaging during wound healing assays and migration assays.
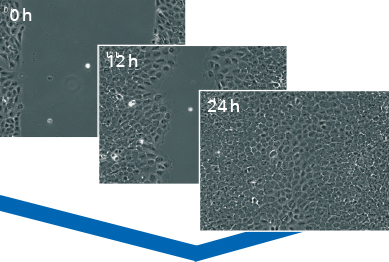
Data Analysis
Procedure
A typical wound healing and migration assay generates gigabytes of imaging data that have to be further processed and analyzed by manual image analysis or by using automated software. In many experimental setups, the main readout is the speed of the wound closure. Other possible readouts are the measurements of the cell-free area and the cell-covered area.
The ibidi Science HubExplore our free online courses and learn more about optimizing your experimental workflows. |
|
Before Starting
The following questions should be addressed before starting the experiment:
Is it possible to reproduce cell culture conditions?
Since many external factors can change the cell behavior, and thereby influence the outcome of a wound healing assay, the cell culture conditions must be consistent. To achieve this, a standard approach should be developed in advance for every wound healing assay. This procedure needs to be kept consistent throughout the experiment. If possible, the same devices, cell passages, number of medium changes, reagents, and disposables should be used for every experiment.
Which cell culture vessel should be used?
Cell culture vessels need to have a thin bottom (~170 µm) that is optimized for high-resolution live cell imaging using inverted microscopy (e.g., the ibidi Polymer Coverslip). The vessel size should be sufficient for convenient gap creation. Depending on the number of samples, either the ibidi Culture-Inserts in the µ-Dish 35 mm, high or the µ-Plate 24 Well are ideal for wound healing assays. In case there is no need for high-resolution fluorescence microscopy, the Culture-Inserts can be self-inserted into a large variety of cell culture formats, such as petri dishes, cell culture chambers, and multiwell plates.
Which substrate should be used?
The substrate depends on the cell type and the experimental conditions being used. For example, a tissue-treated substrate that provides sufficient cell attachment, like the ibidi Polymer Coverslip, works for most cell types. Some cell types, however, require a special coating. In the ibidi Application Note Cell Culture Coating (AN 08) (PDF), you will find detailed information on how to do your own coating on µ-Slides and µ-Dishes.
Is it necessary to inhibit cell proliferation?
During a wound healing assay, cells not only migrate into the gap, but they also proliferate. As this can influence the experimental outcome, proliferation can be inhibited by treating the cells with a proliferation inhibitor, such as mitomycin or actinomycin C, before creating the gap. The dosage and the appropriate controls must be carefully determined in advance so that the cells do not undergo apoptosis or alter their migration capability due to the cytostatic drug.
Which cell density is ideal?
In general, the wound healing assay is done using an optically confluent cell monolayer. Ideally, the assay should always be carried out directly after the monolayer has formed, because the cells might behave differently in matured monolayers. Before starting the experiment, the cell seeding density needs to be defined separately for each cell type. Optimally, the cells should form a monolayer 24 hours after seeding.
How is the gap created?
The consistency of gap creation is highly important when conducting a wound healing assay. Several methods for creating a gap in a cell monolayer have been developed: scratching with a needle or pipet tip, placing an insert or stencil, chemical surface treatment, or the application of electricity. These methods have different advantages and drawbacks, depending on the experimental conditions and the laboratory equipment being used. Find more information about the different methods of gap creation here.
How is the gap closure monitored?
It is possible to observe the gap closure by taking pictures at different time points. However, for most experimental setups, live cell imaging is the most accurate and convenient way to monitor the gap closure.
More Information
Find detailed information about conducting a wound healing assay below:
Instructions
Application Notes
| AN 21: Wound Healing Assay (PDF) |
| AN 36: Wound Healing Assay in µ-Plate 24 Well (PDF) |
Webinar:
| For additional information, please also listen to ibidi’s recorded webinar "Cell Migration, Wound Healing, and Invasion: Performing an Assay and Quantitative Image Analysis". |
Read on and learn more about the Principle of Wound Healing and Migration Assays or different Methods of Creating the Gap.







