Neural Cell Analysis
Neural cell analysis studies the characteristics, functions, and interactions of different cell types that make up the nervous system. The nervous system is composed of various types of cells, including neurons, glial cells, and stem cells, each with distinct roles and functions.
Microscopy is a critical technique for studying neural cells and gaining insight into the nervous system's morphology, structure, and dynamics. While the preparation and imaging of histological samples (e.g., brain slices) were already possible in the late 19th century, the development of advanced imaging techniques over the last 50 years, such as confocal microscopy and 2-photon microscopy, have advanced the visualization of neuronal structures and processes. The combination of new antibodies, fluorescent probes, and labeling techniques has made it possible to depict different neuronal components, and their complex interactive networks, in fixed samples (e.g., in immunofluorescence assays), but also in living cells, tissues, or organisms.
Most recently, with the invention of super-resolution microscopy and its ability to overcome the diffraction barrier of light (~200 nm), structures can be visualized even as small as synaptic vesicles (with a size of 40 nm).
Dynamic cellular processes, such as synapse formation, axon growth and dendritic spine dynamics, can be investigated in real time under physiological conditions using live cell imaging techniques.
In summary, the recent advancements in imaging techniques have allowed for the visualization of neuronal processes and morphology with high spatiotemporal resolution, which play a major role in understanding the function of the brain and nerves.
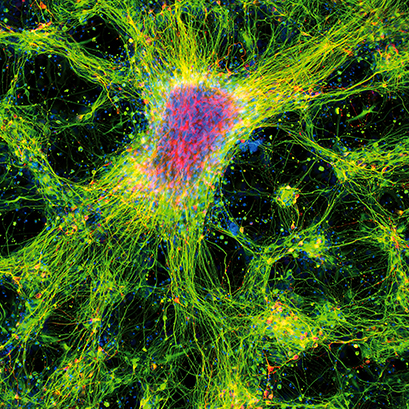
Immunofluorescence image of dopamine neurons derived from human induced pluripotent stem cells (iPSCs) in an ibidi μ-Plate 96 Well. The image shows the neurite extension with the expression of β-III Tubulin (green) and tyrosine hydroxylase (red). DAPI (blue) was used for nuclear staining.
Image Courtesy: Asuka Morizane, Center for iPS Cell Research and Application, Kyoto University, Kyoto, Japan
ibidi Blog Article |
Learn more about Cell-Based Imaging Methods for Investigating the Structure and Function of Neuronal Cells.
Flavia Millesi from the Medical University of Vienna communicates her personal "5 Tips for Preparing Primary Cells from the Peripheral Nervous System for Live Cell Imaging".
ibidi User Protocols
Check out our neuro-related User Protocols:
- UP 07: Protocol for DRG Neuron Preparation (PDF) by Flavia Millesi, Tamara Weiss, Christine Radtke
- UP 12: Time-Lapse Microscopy of Zebrafish Larvae in the ibidi μ-Slide 2 Well Co-Culture (PDF) by Friederike Kessel
- UP 13: Protocol for Immunofluorescent Staining of iPS Cell-Derived Dopaminergic Neurons in the ibidi μ-Plate 96 Well Black (PDF) by Asuka Morizane
ibidi Solutions for Neural Cell Analysis
The ibidi µ-Slides and µ-Dishes include different geometries that combine optimal conditions for everyday cell culture and functional cell-based assays. They are ideal for immunofluorescence, live cell imaging, and high-resolution microscopy. The ibidi labware is available with the ibidi Polymer Coverslip and the ibidi Glass Coverslip. |
|
The ibidi channel slides, especially the µ-Slide VI 0.4, are particularly suitable for immunofluorescence stainings: Their geometry is ideal for the exact exchange of small medium amounts, which is necessary during immunocytochemistry stainings. The channel geometry is ideal for low-volume immunofluorescence assays. |
|
The Chamber Slides, removable are ideal for both low- and high-throughput immunofluorescence experiments and are suitable for the long-term storage of samples that are mounted with a glass coverslip. |
|
The ibidi µ-Plates are ideal for high throughput drug screening and large-scale knock-down and overexpression experiments with high-resolution microscopy as readout. These imaging plates are compatible with robotics and plate readers using the ANSI/SLAS (SBS) standard format. The ibidi µ-Plates have 24, 96, and 384 wells and are available with the ibidi Polymer Coverslip and the ibidi Glass Coverslip with extremely low autofluorescence for undisturbed fluorescence microscopy. |
|
The ibidi Mounting Medium and the ibidi Mounting Medium With DAPI for immunofluorescence have a very low autofluorescence, prevent photobleaching and allow the sample to be stored for several weeks on the µ-Slide without the need for additional coverslips. |
|
The ibidi Stage Top Incubators provide physiological conditions for live cell imaging on every standard inverted microscope. They include CO2 and O2 control (e.g., for hypoxia experiments) as well as actively controlled humidity. They are available for single slides and dishes along with multiwell plates. |
|
User Comments
"The µ-Slide 8 Well high worked very well, especially for our iPSC-derived neurons. We used them for live cell imaging microscopy, and we had very good results. We are very satisfied with the slide!"
Federica Bono, University of Brescia, Italy
"We have tested the µ-Slide 8 Well high Grid-500 as a new option for our long-term experiments. To check the surface with different coating compositions, we plated neuronal progenitor cells (starting from day 16) and cultured them according to our differentiation protocol. We were pleased to find that the cells are now in culture beyond day 60 and have developed magnificently. We noticed that both the Vitrogel and Geltrex coating have formed a stable bond with the surface of the slide and have not detached from it."
Robin Friedrich, Hamm-Lippstadt University of Applied Sciences, Germany
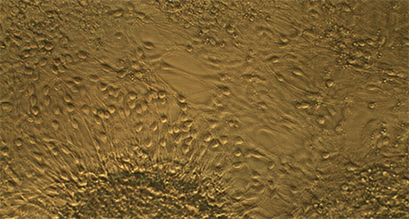
Live cell imaging image of neuronal progenitor cells in an ibidi µ-Slide 8 Well high Grid-500. The image shows differentiated neurons on day 50.
Image Courtesy: Robin Friedrich, Hamm-Lippstadt University of Applied Sciences, Germany
Selected References
Immunofluorescence of Neuronal Cells
The µ-Slide 8 Well was used for the cultivation and immunostaining of rat Schwann cells (rSC), fibroblasts (rFB), and dorsal root ganglion (rDRG) neurons.
Millesi F, Weiss T, Mann A, Haertinger M, Semmler L, Supper P, Pils D, Naghilou A, Radtke C. Defining the regenerative effects of native spider silk fibers on primary Schwann cells, sensory neurons, and nerve-associated fibroblasts. FASEB J. 2021 Feb;35(2):e21196. doi: 10.1096/fj.202001447R.
Read article
Imaging of Neuronal CAD Cells
The µ-Dish 35 mm, high was used to culture and immunostain neuronal CAD (Cath.a-differentiated) cells to quantify tunneling nanotubes connected cells (TNT) and filopodia connected cells, as well as to image the transfer of DiD-labelled vesicles between two cell populations.
Bhat S, Ljubojevic N, Zhu S, Fukuda M, Echard A, Zurzolo C. Rab35 and its effectors promote formation of tunneling nanotubes in neuronal cells. Sci Rep. 2020 Oct 8;10(1):16803. doi: 10.1038/s41598-020-74013-z.
Read article
Live Cell Imaging of Cortical Neurons
The µ-Slide 8 Well was used for confocal live cell imaging of Purkinje neurons.
Motori E, Atanassov I, Kochan SMV, Folz-Donahue K, Sakthivelu V, Giavalisco P, Toni N, Puyal J, Larsson NG. Neuronal metabolic rewiring promotes resilience to neurodegeneration caused by mitochondrial dysfunction. Sci Adv. 2020 Aug 28;6(35):eaba8271. doi: 10.1126/sciadv.aba8271.
Read article
Histology of Brain Slices and Spinal Cords
Brains and spinal cords were dissected from adult mice for immunohistostaining. The ibidi Mounting Medium was added in preparation for imaging.
Usseglio G, Gatier E, Heuzé A, Hérent C, Bouvier J. Control of Orienting Movements and Locomotion by Projection-Defined Subsets of Brainstem V2a Neurons. Curr Biol. 2020 Dec 7;30(23):4665-4681.e6. doi: 10.1016/j.cub.2020.09.014.
Read article
Read on and learn more about Neurological Conditions, Disorders, and Diseases, Neural 3D Cell Models, and Neural Function and Behavior.