Confocal Microscopy:
Principle, Applications, and ibidi Solutions
What Is Confocal Microscopy?
Confocal laser scanning microscopy (CLSM)—also referred to as confocal microscopy—is an advanced fluorescence imaging technique that enables high-resolution, three-dimensional visualization of biological samples. It is widely used in cell biology, neuroscience, developmental biology, and cancer research to analyze both fixed and living cells in 2D and 3D.
The key innovations of CLSM are the use of point-scanning laser excitation and a pinhole aperture, which together suppress out-of-focus fluorescence. This optical sectioning capability enhances image clarity and contrast, allowing researchers to focus on specific z-planes within a thick sample.
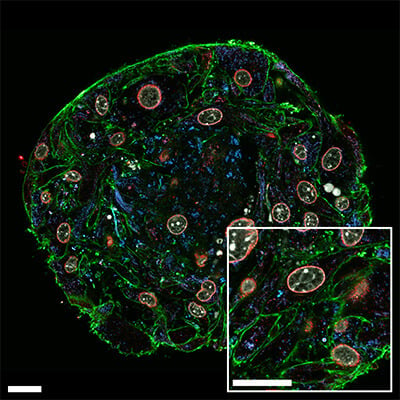
Confocal image of a NIH3T3 spheroid grown on the µ-Slide VI 0.4 µ-Pattern ibiTreat, cir200, pit600, hex, labeled with abberior dyes and imaged with the abberior STEDYCON. Scale bar: 20 µm.
How Does Confocal Microscopy Work?
Laser Scanning and Optical Sectioning
In confocal laser scanning microscopy, a focused laser beam scans the sample in a raster pattern. Unlike widefield fluorescence microscopy, which illuminates the entire sample, CLSM uses highly focused laser light to excite fluorophores within a confined focal volume. The emission fluorescence light, originating only from the focal plane, is then directed through a pinhole aperture in the optical path, which further blocks out-of-focus signals, resulting in sharper, high-contrast images.
By acquiring a series of images along the z-axis, also called z-stack imaging, researchers can reconstruct three-dimensional (3D) structures of cells, spheroids/organoids, or tissue slices. Afterwards, deconvolution techniques can enhance image fidelity by computationally compensating for optical aberrations and improving the apparent signal-to-noise ratio, thereby reducing image blur introduced by the imaging system.
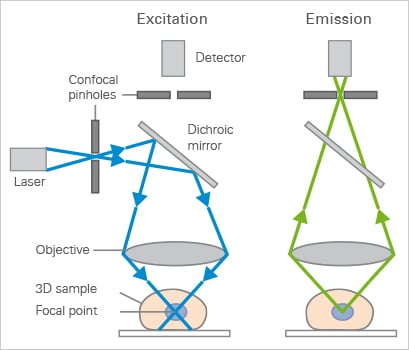
Excitation (left) and emission (right) light path in a basic confocal microscope setup.
Multicolor Imaging and Spectral Unmixing
Modern CLSM systems support multichannel fluorescence detection. By combining multiple laser lines or using spectral unmixing, multiple biomolecules can be detected simultaneously—ideal for colocalization and interaction studies (e.g., FRET, FLIM).
Applications in Biological Research
Confocal microscopy is indispensable for various applications such as cell biology, neuroscience, cancer research, and other life science fields to study detailed cellular structures in both fixed and living cell imaging experiments. With its ability to perform high-resolution fluorescence images, researchers can label specific cell structures or proteins using immunofluorescence techniques and visualize them with exceptional clarity. One key application of confocal microscopy is the ability to perform 3D volume scans of thick biological samples, such as organoids, spheroids, and tissue sections, by eliminating out-of-focus light through optical sectioning.
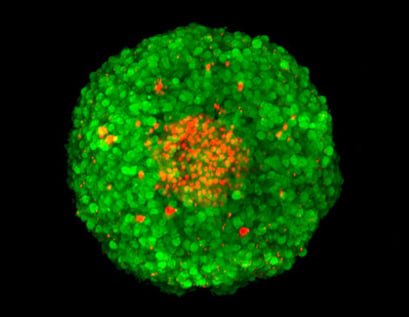
Z-stack image of an FDA/PI-stained MCF-7 spheroid, acquired using confocal microscopy. Green: FDA-stained living cells. Red: PI-stained dead cells in the necrotic center of the spheroid.
Limitations and Complementary Techniques
Confocal microscopy has limitations in light penetration depth (typically up to 100 µm). Moreover, it can cause strong photobleaching or even phototoxicity effects—a factor that is especially important when imaging living samples. For larger samples, in vivo imaging or whole-organ visualization, alternative techniques such as two-photon microscopy, light sheet fluorescence microscopy (LSFM), or proper sample preparation techniques such as tissue clearing protocols are needed.
ibidi Solutions for Confocal ImagingAll ibidi µ-Slides, µ-Dishes, and µ-Plates are fully compatible with confocal and high-resolution fluorescence microscopy. The ibidi #1.5 Polymer Coverslip and the ibidi #1.5H Glass Coverslip provide ideal optical conditions for confocal microscopy. |
|





