Fluorescence Lifetime Imaging Microscopy (FLIM)
Applications:
FLIM is used for analyzing the distribution of specific cellular components, such as proteins or nucleic acids. It can be used to gain information about the molecules and to visualize the state of the environment that surrounds the respective molecule in living cells. FLIM helps to measure several factors, such as calcium ion amount, pH, oxygen concentration, molecular interaction, and molecular binding. Under optimal conditions, it is even possible to detect and identify single molecules. The penetration depth using FLIM is higher compared to standard fluorescence microscopy, which enables the analysis of thicker samples.
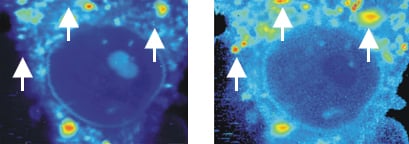
Live cell imaging of the fluorescence intensity (left) and fluorescence lifetime (FLIM, right) of a mouse fibroblast in a rhodamine 800 solution. Red color indicates higher fluorescence intensity or longer fluorescence lifetime, respectively. Note the low fluorescence intensity but high fluorescence lifetime in several areas, indicated by white arrowheads.
Principle:
In contrast to standard fluorescence microscopy where the intensity is used to create an image of the specimen, FLIM uses the lifetime of the signal by analyzing the fluorophore’s exponential decay rate. The fluorescence lifetime is specified as the average time that a fluorophore stays in the excited state before emitting a photon and returning to the ground state. In the excited state, each type of fluorophore has its own lifetime. By detecting differences in lifetime, it is possible to distinguish fluorophores that have the same excitation and emission spectrum.
The fluorescence lifetime depends on the local environment. While it is affected by factors such as molecular interaction or ion concentration, it is not influenced by fluorophore concentration, photobleaching, or excitation light intensity. The resulting FLIM image contrasts the lifetime of the individual fluorophores, which can then be used to define and interpret the environmental factors of the molecules of interest. The lifetime also changes when an energy acceptor molecule is in close proximity. This combination of FLIM and FRET gives detailed insight into sub-molecular binding processes.
H.C. Ishikawa-Ankerhold, R. Ankerhold, G.P.C. Drummen. Advanced Fluorescence Microscopy Techniques—FRAP, FLIP, FLAP, FRET and FLIM. Molecules, 2012, 10.3390/molecules17044047
read abstract
ibidi Solutions:
|  |




