Two-Photon and Multiphoton Microscopy
Applications:
Two-photon microscopy (also called multiphoton microscopy) can be used for live cell imaging of thick biological specimens, as it has several advantages over confocal microscopy. Molecules can be visualized deeply within the specimen with a maximal penetration depth of about 1 mm. This enables 3D imaging of tissue slices, organoids, whole organs, embryos, or even whole animals.
In contrast to confocal microscopy, two-photon microscopy works with higher wavelengths leading to less photobleaching or photodamage, which is especially important when working with living samples.
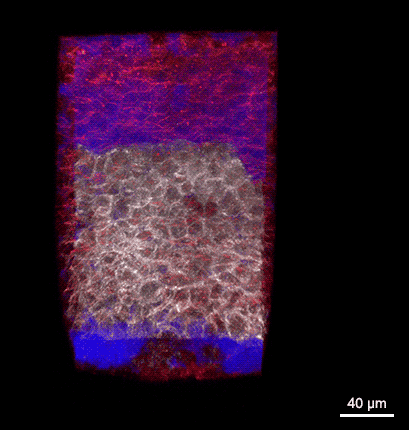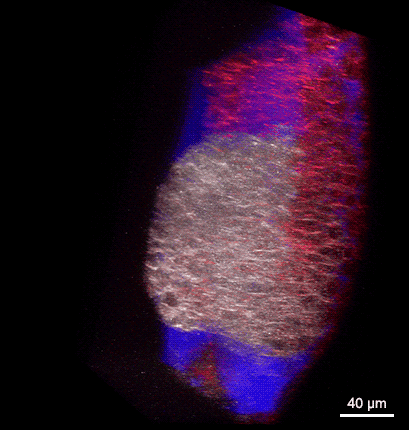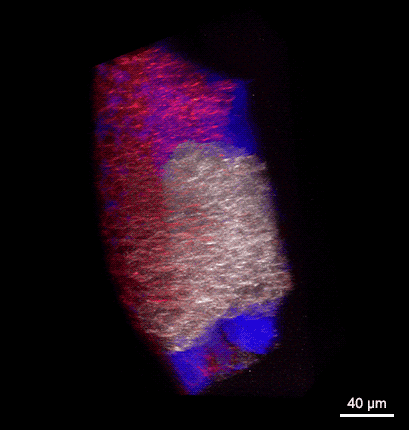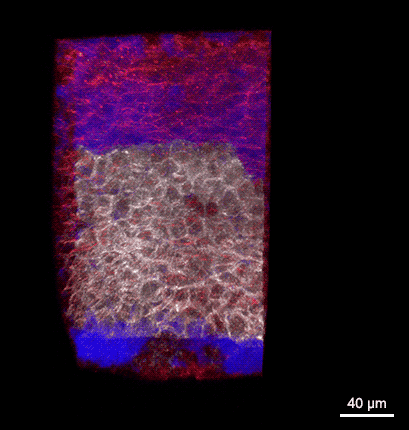
Rotating 3D rendering of a confocal image stack showing the dorsal germ ring of a zebrafish embryo at the onset of gastrulation (6 hours post fertilization). GFP (white) is expressed in internalizing prechordal plate progenitors and lyn-TagBFP (red) marks the membrane in all cells. The embryo was injected with dextran-rhodamine to label the interstitial fluid (blue). The image was recorded at the Bioimaging Facility of the Institute of Science and Technology Austria (IST), using a multiphoton LaVision BioTec TriM Scope microscope.
Principle:
Just like widefield or confocal fluorescence microscopy, two-photon microscopy is based on fluorophore excitation, which results in the emission of light. In classic fluorescence microscopy, a fluorophore is excited by absorbing one single photon of a certain wavelength. When using two-photon microscopy, two or three photons of a higher wavelength do the work of one: When they hit the fluorophore at the very same time (typically within several femtoseconds), they are absorbed, resulting in fluorophore excitation and emission of light.
In this process, photons combine their energy, which allows low-energy infrared photons to excite standard fluorophores, such as GFP. The infrared light penetrates tissue more deeply than the standard excitation light used in fluorescence microscopy. Due to its low energy level, infrared light is less damaging, and therefore especially useful when working with living samples.
In order to increase the likelihood that two photons hit the fluorophore simultaneously, lasers with very high intensity are needed. Their infrared light only leads to excitation in the focus of the objective, because only in this area, the critical number of photons per time and space is reached. Therefore, all emitted light comes from one focal point in the specimen, strongly reducing background noise. The image is created just as in confocal microscopy: The laser scans across the sample, recording the image intensity point by point.
R.K.P. Benninger, D.W. Piston. Two-photon excitation microscopy for the study of living cells and tissues. Curr Protoc Cell Biol, 2013, 10.1002/0471143030.cb0411s59
read abstract
ibidi Solutions:
|  |




