Application Notes in Numerical Order
AN 01: Gradients Inside µ-Slide I (PDF)
Establishing a concentration profile
AN 02: Fluorescence Staining Using a µ-Slide I (PDF)
Examples describing how to do immunofluorescence stainings using µ-Slides
AN 03: Cell Culture in ibidi Channel Slides: Using the µ-Slide VI 0.4 as an Example (PDF)
Benefits and handling of the µ-Slide VI 0.4, and a comparison between channel slides and slides with open wells
AN 05: Tube Formation Assay in the µ-Plate 96 Well 3D (PDF)
Handling protocol for tube formation assays using a multi-channel pipette and the µ-Plate 96 Well 3D
AN 06: Cultivation and Detachment of Adherent Cells in the µ-Slide VI 0.4 (PDF)
Removing adherently grown cells from a µ-Channel after cultivation
AN 07: Transfection (PDF)
Example showing how protocols for transfection can easily be adapted to the work in cell culture channels
AN 08: Coating Protocols for ibidi Labware (PDF)
A detailed protocol on how to coat the ibidi labware (e.g., with Collagen, Fibronectin, or Poly-L-Lysine) including required volumes and protein concentrations
AN 09: Fluorescence Staining Using a µ-Slide VI (PDF)
Examples describing how to do immunofluorescence stainings using µ-Slides
AN 10: Co-Cultivation (PDF)
Application note for co-cultivation of two different cell types in µ-Slide 2 Well Co-Culture
AN 11: Shear Stress and Shear Rate Calculations for ibidi µ-Slides (PDF)
Detailed information on how to calculate shear stress, shear rates, and flow rates in the ibidi channel slides
AN 12: Avoiding Evaporation: Humidity Control in Cell Culture (PDF)
An overview of different methods to avoid evaporation from cell culture vessels by controlling the humidity
AN 13: Endothelial Cells Under Perfusion (PDF)
Setting up a flow experiment using μ-Slide I and HUVECs
AN 14: Live Cell Imaging Under Flow (PDF)
Discussion of various setups for live cell imaging experiments under flow conditions
AN 15: Fluorescence Staining Using the µ-Slide y-shaped (PDF)
Examples describing how to do immunofluorescence stainings using µ-Slides
AN 16: Immunofluorescence Staining Using the µ-Slide 8 Well high (PDF)
Detailed information on how to perform an immunofluorescence (IF) staining in the µ-Slide 8 Well high
AN 18: Shear Stress and Shear Rate Calculations for the µ-Slide y-shaped (PDF)
Detailed information on how to calculate shear stress, shear rates, and flow rates in the µ-Slide y-shaped
AN 19: Tube Formation Assay in the µ-Slide 15 Well 3D (PDF)
Setting up a tube formation assay with the µ-Slide 15 Well 3D
AN 20: Cultivation of Macrophages (PDF)
Cultivation protocol for a murine macrophage cell line in µ-Slide VI 0.4 and µ-Slide 8 Well
AN 21: Wound Healing Assay Using the ibidi Culture-Insert 2 Well in a µ-Dish 35 mm, high (PDF)
Setting up a wound healing assay with the ibidi Culture-Insert in a µ-Dish 35 mm, high
AN 22: Determination of the Pixel Size in Microscopy Images (PDF)
Measuring and calculating the pixel size of microscopic images
AN 23: 3D Chemotaxis Protocol for Non-Adherent Cells in a Gel Matrix (PDF)
Application Note providing a specific example protocol for chemotaxis of dendritic cells in a collagen gel
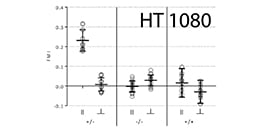
AN 24: Chemotaxis of HT-1080 Cells in 2D and 3D (PDF)
Detailed protocol of experimental parameters and example data of HT-1080 cells migrating in 2D and in 3D in collagen I gels
AN 25: Serial Connection of Luer-Slides for Flow Experiments (PDF)
Step-by-step protocol for how to create a serial connection between two µ-Slide I 0.6 Luer channels
AN 26: Collagen I Gel for 3D Cell Culture (PDF)
Fabrication protocols for collagen I gel (bovine and rat tail) with different cell media
AN 27: Optimizing Tube Formation Assays (PDF)
Experimental setup optimization of tube formation assays
AN 30: Optimizing Wound Healing and Cell Migration Assays (PDF)
Experimental setup optimization of wound healing assays and cell migration assays
AN 31: Serial Connection of µ-Slide VI 0.4 Channels for Flow Experiments (PDF)
Step-by-step protocol for how to create a serial connection between the six channels of the µ-Slide VI 0.4
AN 32: Generation of Spheroids (PDF)
Generation of spheroids using the liquid overlay technique
AN 33: Live / Dead Staining with FDA and PI (PDF)
Viability staining of adherent cells, single cells embedded in extracellular matrix, and cellular clusters
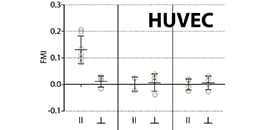
AN 34: Chemotaxis of HUVECs in 2D and 3D (PDF)
Detailed protocol of experimental parameters and example data of HUVECs migrating in 2D and in 3D in collagen I gels.
AN 35: Chemotaxis Assay with Cells Producing a Chemoattractant (PDF)
A detailed protocol for using adherent cells as chemoattractant-producers in the large reservoirs of the µ-Slide Chemotaxis.
AN 36: Wound Healing Assay in µ-Plate 24 Well (PDF)
A handling protocol for wound healing assays: Screening substances for pro- or anti-migrational effects
AN 37: Image Shift Correction in Microscopic Time Lapse Sequences (PDF)
Instructions for correcting an externally generated shift of the sample in time lapse images (e.g. in chemotaxis experiments)
AN 38: Western Blot Analysis with Cell Samples Grown in Channel-µ-Slides (PDF)
Detailed protocol for performing cell lysis in channel-µ-Slides and subsequent SDS-Page with Western Blot Analysis
AN 40: Gene Expression Profiling with Cell Samples Grown in Channel-µ-Slides (PDF)
Detailed protocol for performing cell lysis in channel-µ-Slides and subsequent RNA-Isolation with RT-qPCR Analysis
AN 44: Immunofluorescence Staining of HUVEC in 3D in the μ-Slide Chemotaxis (PDF)
A detailed protocol for immunostaining of HUVEC in a gel matrix in the µ-Slide Chemotaxis.
AN 45: Mounting Medium Types (PDF)
A comparison of non-hardening and hardening mounting media
AN 49: Fluorescence Staining Using a 12 Well Chamber, Removable (PDF)
A handling protocol for immunofluorescence staining in the 12 Well Chamber, removable
AN 50: Fluorescence Staining Using a 3 Well Chamber, Removable (PDF)
A handling protocol for immunofluorescence staining in the 3 Well Chamber, removable, including instructions for using the volume-minimizing coverslip
AN 58: Immunofluorescence Staining Using the μ-Slide 18 Well (PDF)
Example for an immunofluorescence staining in the µ-Slide 18 Well.
AN 59: Human Endothelial Cells Cultivated Under Shear Stress on the Glass Membrane of the µ-Slide ibiPore SiN (PDF)
Instructions on how to cultivate HUVECs on the µ-Slide ibiPore SiN under shear stress by using the ibidi Pump System.
AN 60: HUVECs Under Shear Stress on a Collagen Matrix in the μ-Slide I Luer 3D (PDF)
Culture of human endothelial cells under shear stress on a collagen matrix in the μ-Slide I Luer 3D
AN 61: Immunofluorescence Staining of HUVECs on a Gel Matrix Using the µ-Slide I Luer 3D (PDF)
Immunofluorescence staining of human endothelial cells on a gel matrix using the μ-Slide I Luer 3D
AN 63: Generation and Dynamic Culture of L929 Spheroids in the µ-Slide Spheroid Perfusion (PDF)
Protocol for creating multicellular spheroids in the µ-Slide Spheroid Perfusion with subsequent flow application
AN 64: FDA/PI Live/Dead Staining Using L929 Spheroids in the µ Slide Spheroid Perfusion (PDF)
Protocol for an FDA/PI fluorescence staining in the µ-Slide Spheroid Perfusion to distinguish living and dead cells in a spheroid
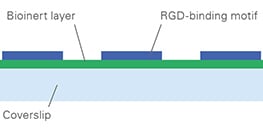
AN 65: Cell Adhesion on ibidi μ-Patterns: Parameters and Optimization (PDF)
A detailed protocol on how to test and optimize cell adhesion on the ibidi µ-Patterns
AN 66: Tube Formation Assay With Laminin-Collagen I Gel in the μ-Slide 15 Well 3D (PDF)
A detailed protocol for performing a tube formation assay with a Laminin-Collagen I gel (instead of Matrigel®) in the μ-Slide 15 Well 3D
AN 67: Data Analysis of Wound Healing and Cell Migration Assays (PDF)
Methods for data analysis of wound healing assays and cell migration assays
AN 68: Co-Culture of Tumor Spheroids and HUVEC Under Flow Using the μ-Slide I Luer 3D (PDF)
A protocol and example of co-cultivation of tumor spheroids with a monolayer of HUVEC cells under perfusion using the ibidi Pump System
AN 69: Serial Connection of μ-Slide Spheroid Perfusion Channels for Long-Term Cultivation of 3D Spheroids Under Flow (PDF)
A detailed protocol to connect multiple µ-Channel Slides in series using one Pump System
AN 70: Data Analysis of Tube Formation Assays (PDF)
Protocol for data analysis of tube formation assays
AN 71: Co-Culture of Tumor Spheroids and HUVEC Under Flow Using the μ-Slide III 3D Perfusion (PDF)
A protocol and example of co-cultivation of tumor spheroids with a monolayer of HUVEC cells under perfusion using the ibidi Pump System
AN 72: RGD Micropatterning Using the ibidi Micro Illumination System for Spheroid Generation and Cultivation (PDF)
A protocol for light-induced micropatterning by click chemistry for the generation of spheroids
AN 73: 2D Whole Protein Pattern Based on a PLL-PEG-Passivated Coverslip Surface Using the ibidi Micro Illumination System (PDF)
A protocol for different surface micropatterns directly into the µ-Dish
AN 74: 3D Hydrogel Constriction in the µ-Slide I Luer Using the ibidi Micro Illumination System (PDF)
A protocol to create a perfused microchip used as model for arteriosclerosis
AN 75: Structuring a Photoresist-Coated Wafer With Photolithography Using the ibidi Micro Illumination System (PDF)
A protocol for soft photolithography
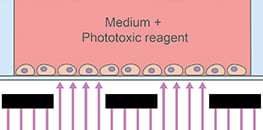
AN 76: Photo-Induced Cell Migration Using the ibidi Micro Illumination System (PDF)
A protocol for a light-induced wound healing assay.
AN 77: Co-Culture Invasion Assay Using the Culture-Insert 2 Well and the Live Cell Labeling Kit CellarisTM (PDF)
A protocol for co-cultivation with transient but highly stable labeled cells using the Cellaris dyes
AN 78: Cell Culture and Immunofluorescence Staining in the μ-Slide VI 0.4 μ-Pattern ibiTreat (PDF)
A protocol for the cultivation, fixation, and staining of cells or spheroids on micropatterns
AN 79: Selective and Localized Cell Adhesion using a CD19-Coated Custom µ-Pattern ibiTreat (PDF)
CD19 antibody coating on an ibidi micropattern to selectively capture CD19-positive Nalm-6 cells from a mixed cell population
AN 80: Formation and Long-Term Cultivation of Spheroids in the μ-Slide 8 Well high μ-Pattern ibiTreat (PDF)
Step-by-step protocol on how to create spheroids on ibidi micropatterns
Application Note 81: Photomask Layout Creation for the ibidi Micro Illumination System (PDF)
Step-by-step protocol on how to create a photomask for the Micro Illumination System