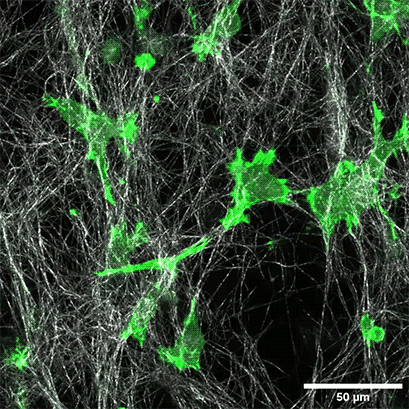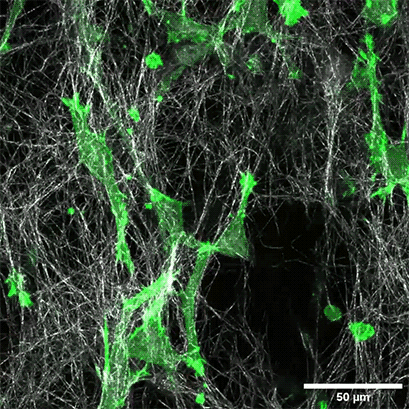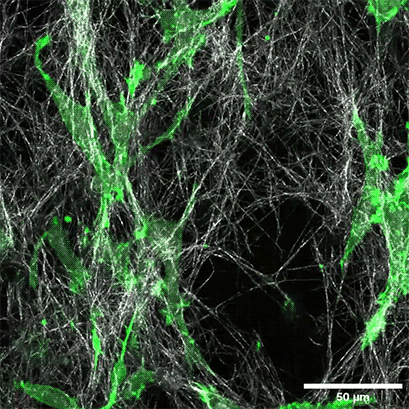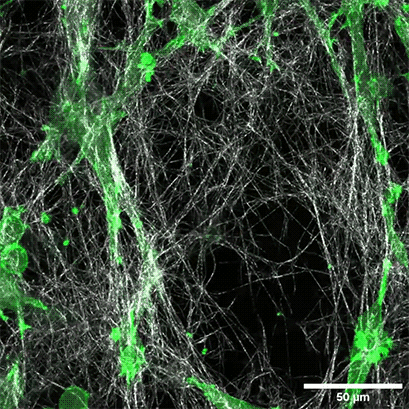
LifeAct-expressing HT-1080 cells (green) were seeded in a 1.5 mg/ml Collagen Type I, Rat Tail layer (white) in the µ-Slide Chemotaxis. Cell migration was documented by taking a photo every 300 seconds on a Zeiss Confocal Microscope LSM 880 AxioObserver using a water immersion objective lens 40x/1.2.
![]()
Learn how it was done in our Generation and Cultivation of 3D Cell Models chapter.
Spheroid and Organoid Culture
Spheroid Formation on a Defined Micropattern
Micropatterns are powerful tools for optimizing 3D assays. ibidi's µ-Patterns ibiTreat with multi-cell patterns enable spatially defined cell adhesion for various 2D and 3D cell culture applications. Defined adhesion spots, surrounded by Bioinert, are able to catch adherent single cells from a cell suspension. Bioinert is fully non-cell-attachable. This forces all cells to aggregate to each other at the adhesion spots, thus forming spheroids in a defined and controllable way.
![]()
Read about the technology behind controlled cell adhesion in our Micropatterning Application Chapter.
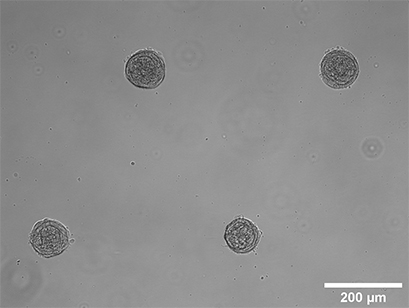
Spheroid formation of NIH-3T3 cells (murine embryo fibroblasts). Image taken 14 days after seeding single cells in a µ-Slide 8 high Well with multi-cell µ-Patterns. Brightfield microscopy, 10x objective lens.
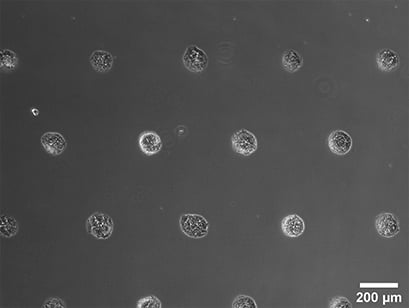
Spheroid formation of NIH-3T3 cells (murine embryo fibroblasts), 14 days after seeding single cells in the µ-Slide VI 0.4 with multi-cell µ-Patterns. Phase contrast microscopy, 4x objective lens.
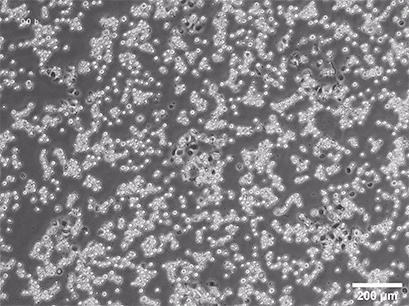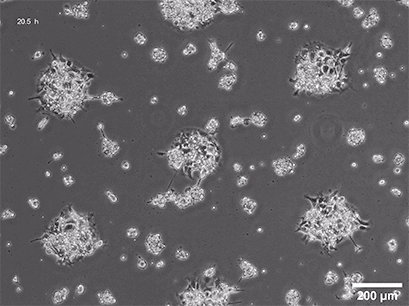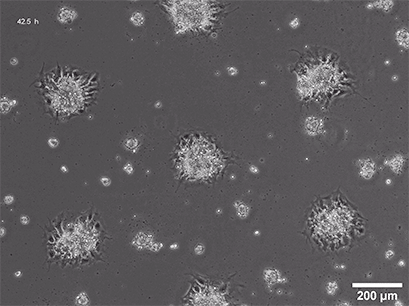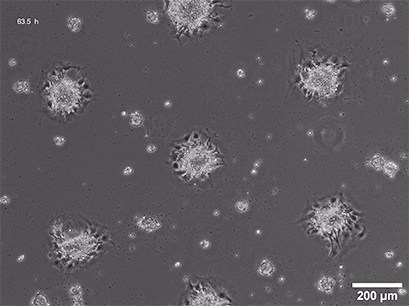
Spheroid formation of NIH-3T3 cells (murine embryo fibroblasts) on 200 µm adhesion spots using µ-Patterning. Spheroid generation was documented for 64 hours. Phase contrast live cell imaging, 4x objective lens.
![]()
Learn how it was done in our Generation and Cultivation of 3D Cell Models chapter.
Highly Improved Spheroid Growth Rates When Cultured Under Perfusion

L929 fibroblasts show spheroid formation in the µ-Slide Spheroid Perfusion, Bioinert, days 1–14, seeding concentration 5 x 105 single cells/ml. Left: no perfusion, medium exchange every second day. Right: perfusion with the ibidi Pump System, 0.75 ml/min. Phase contrast microscopy, 10x objective lens, well diameter 800 µm.
Live/Dead Staining of a Long-Term Cultured Spheroid
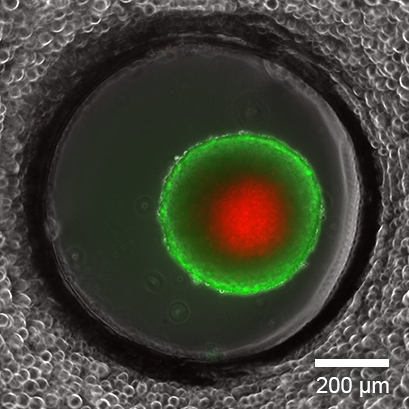
Live/dead FDA/PI staining of an L929 spheroid in the µ-Slide Spheroid Perfusion, Bioinert, after 14 days in culture with perfusion using the ibidi Pump System, 0.75 ml/min. Green: living cells (fluorescein diacetate, FDA); red: dead cells (propidium iodide, PI). Widefield fluorescence microscopy, 10x objective lens.
Organoid Co-Culture of PDAC Cells and
Fibroblasts
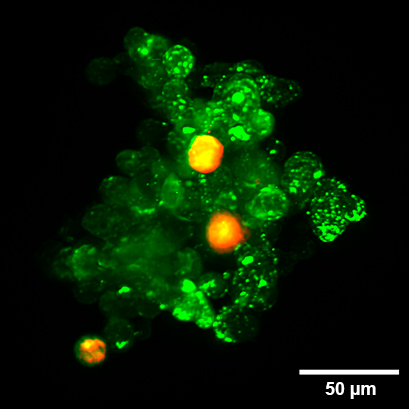
Organoid co-culture of the human pancreatic cancer (PDAC) cell line PA-TU-8988T (green, stained with CellTrackerTM Green) and the murine fibroblast cell line mPSC4 (red, stained with CellTrackerTM Orange CMTMR) in the µ-Slide Spheroid Perfusion. The µ-Slide was covered with a 25 µm FEP foil for matching the refractive index closer to water during upright light sheet microscopy. The image was acquired by S. Volkery at MPI Muenster with the M Squared Aurora Airy beam upright light sheet setup. The sample was provided by K. Roth, University Marburg, Germany.
Invasion of HT-1080 Cancer Cells
in a 3D Collagen Gel
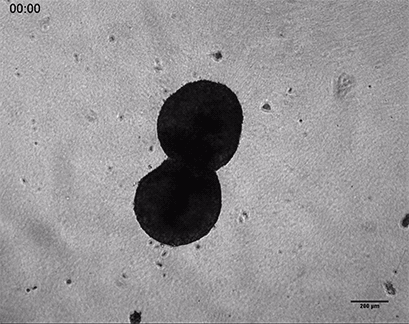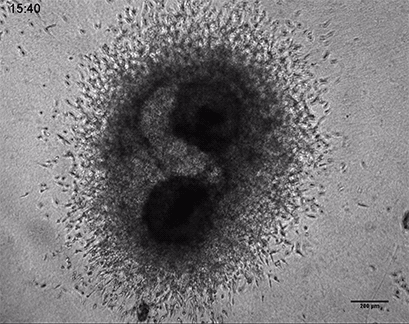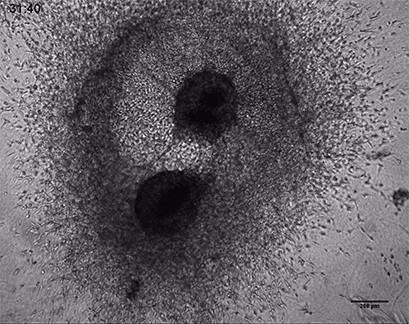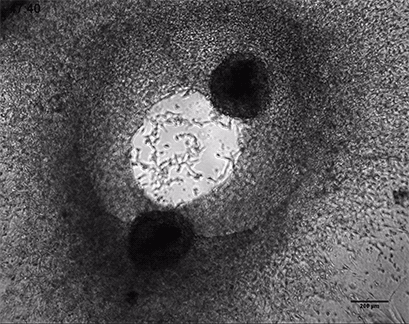
Invasive human fibrosarcoma cancer spheroids (HT-1080) were embedded into Collagen Type I, Rat Tail gel. The invasion into the gel matrix was recorded for 48 hours in the µ-Slide 8 Well. 4x objective lens, brightfield.
Sprouting of Endothelial Cells
in a 3D Collagen Gel
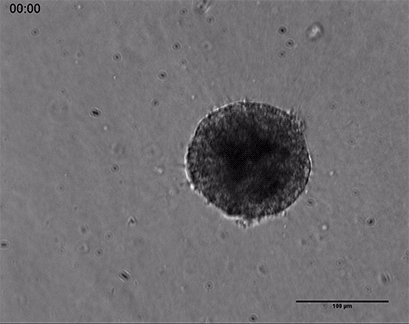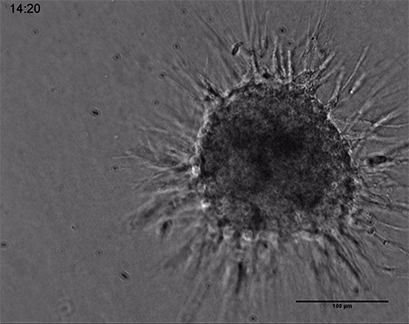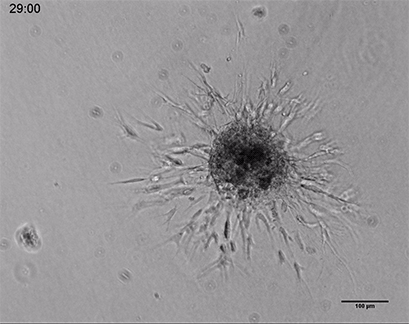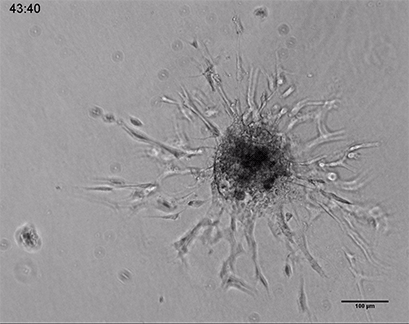
Live cell imaging of a spheroid of Human Umbilical Vein Endothelial Cells (HUVEC), embedded into a 3D gel made of Collagen Type I, Rat Tail. The sprouting process into the gel matrix was recorded for 44 hours in the µ-Slide 8 Well. 10x and 4x objective lens, brightfield.
Confocal Microscopy of a Cross-Section of an L929 Spheroid
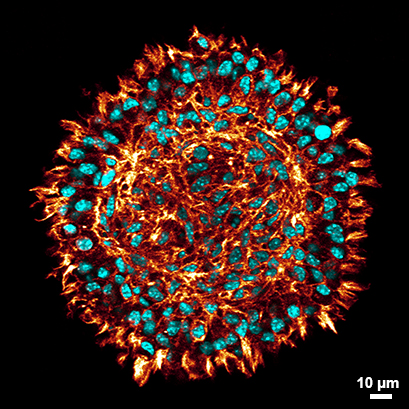
Confocal microscopy of a cross-section of a stained L929 spheroid after clearing (red: phalloidin, cyan: DAPI) in the µ-Slide Spheroid Perfusion. Clearing of the spheroids allows the visualization of cells even at the center of the spheroid fibroblasts while at the same time preserving the spheroid`s morphology. Data by Julian Hofmann and Selina J. Keppler, Technical University Munich, Germany.
![]()
Find detailed information in the User Protocol 11: “Protocol for Spheroid Culture, Staining, and Clearing for 3D Imaging".
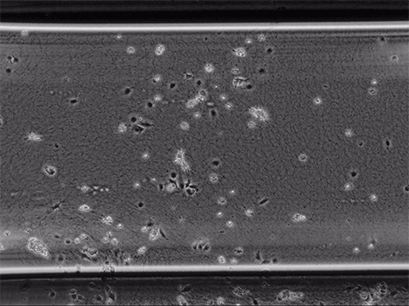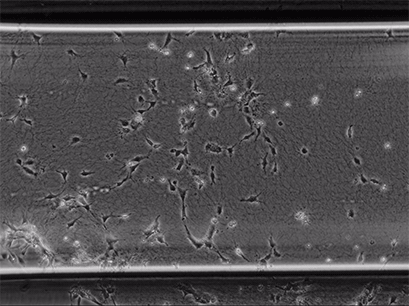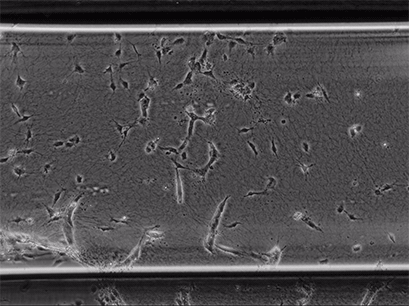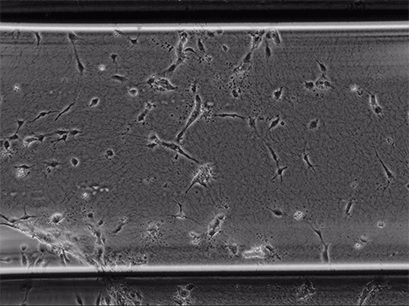
Live cell imaging of Human Umbilical Vein Endothelial Cells (HUVEC) embedded in a 1.5 µg/ml Collagen Type I gel in the µ-Slide Chemotaxis, migrating towards fetal calf serum. Note: the cells connecting to each other form strings during the chemotaxis process. Phase contrast, 4x objective lens, 24 hours.
![]()
Learn about the chemotaxis experiment workflow including data analysis in our Chemotaxis Assays Application Chapter.




