TT3: PTubeChip – 3D-Printed Organ Models with Perfused Microchannels
The PTubeChip is a microfluidic platform that enables the dynamic cultivation of cells inside 3D-printed hydrogel channels.
With the PTubeChip, ibidi and its partners bring together microfluidic precision and 3D bioprinting to enable dynamic organ-on-chip models for biomedical research.
Why It Matters
For the first time, the PTubeChip makes it possible to combine 3D bioprinting and microfluidics in a single, perfused system, which allows researchers to grow living tissues that closely mimic real human organs. This breakthrough opens a new generation of organ-on-chip models that can reproduce complex disease mechanisms and accelerate medical progress.
These realistic, perfused organ models give new opportunities for drug discovery, toxicology, and personalized medicine to treat diseases such as cancer, atherosclerosis, stroke, and acute or chronic kidney disease.
PTubeChip helps researchers to:
- Reduce the need for animal testing
- Provide more predictive, human-based data
- Enable research on patient-derived organ models
The PTubeChip represents a major step toward replacing animal experiments and developing patient-specific organ models that could transform the way therapies are discovered and tested.
The Idea Behind the Technology: A New Approach to Organ-on-Chip Development
The PTubeChip is a microfluidic platform that enables the dynamic cultivation of cells inside 3D-printed hydrogel channels. Originally developed in collaboration with CELLINK (Gothenburg) and the Natural and Medical Sciences Institute (NMI) at the University of Tübingen, this prototype demonstrates ibidi’s pioneering role in bridging 3D bioprinting and microfluidics.
By using sacrificial 3D-printed structures, the PTubeChip allows the formation of hollow channels within soft hydrogels that can be connected to a perfusion system via Luer ports. These channels can then be lined with endothelial or epithelial cells, creating realistic biological barriers that mimic organ functions such as the proximal tubule of the kidney.
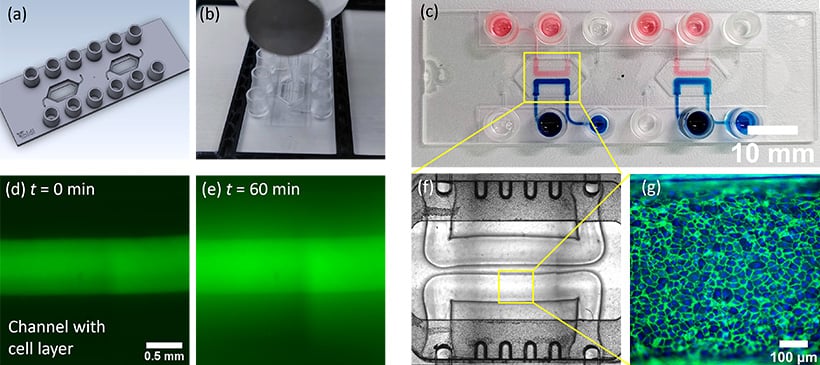
(A) Digital image of the microfluidic chip with two chambers for 3D-bioprinting. (B) Printing process of channels in the microfluidic chip. (C) Microfluidic chip with two channels printed in each chamber. The channels are filled with either blue or red-colored liquid to confirm that no leaking or mixing occurs. (D) Test of monolayer formation of primary endothelial cells inside 3D-printed channels. A channel was cultivated with endothelial cells until they formed a confluent monolayer. The channel was then filled with a solution containing fluorescent dye. The two images show the fluorescence signal before (D) and after (E) 60 min incubation with the dye, indicating a reduced diffusion of the dye through the monolayer. (F) A microscopy image of two channels within the microfluidic chip. The channel diameter is about 500 µm and the height is 200 µm. (G) Primary endothelial cells seeded and cultivated for one week under perfusion in the channel. The image shows fluorescence staining of nuclei (blue) and the cell junction protein PECAM1 (green).
Status and Outlook
While the original research project has been completed, further chip development continues as a collaboration between ibidi and CELLINK. The PTubeChip is currently at the prototype stage and protected under patent application EP4268958A1 (published 01.11.2023).
Synergies and Compatibility
The PTubeChip integrates seamlessly with the ibidi Pump System and CELLINK’s BioX 3D Bioprinter, combining microfluidic precision with bioprinting flexibility.
Related ibidi Products
Key Facts
- Partners: CELLINK (Gothenburg), NMI Tübingen
- Project lead: Philipp Linke (plinke@ibidi.de)
- Related patent applications or patents: EP4268958A1 (01.11.2023)
- Stage: Prototype
- Focus Area: Organ-on-Chip, 3D Bioprinting, Microfluidics, Angiogenesis




