Immune Cells Under Shear Stress
Immune cell migration is a critical process in the immune response to infection. In order to mount an effective response against pathogens, immune cells must first migrate from the blood to the site of infection. This process is facilitated by a combination of chemical signals, including cytokines and chemokines, which are released by infected or damaged tissues.
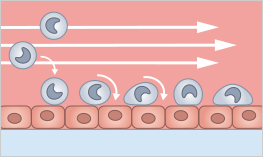
Rolling and adhesion are key steps in the process of immune cell migration. When immune cells encounter the chemical signals produced by an infected or damaged tissue, they roll along the inner surface of the blood vessels. This rolling is facilitated by selectin molecules on the surface of both the immune cells and the endothelial cells that line the blood vessels.
Once the immune cells have slowed down, they are able to adhere firmly to the endothelial cells through the interaction of integrin molecules on the surface of the immune cells and adhesion molecules on the surface of the endothelial cells. This adhesion allows the immune cells to crawl through the vessel wall and into the surrounding tissue, where they can phagocytose pathogens and initiate the immune response.
Rolling and adhesion are tightly regulated processes, and the failure of immune cells to effectively roll and adhere can impair the ability of the immune system to fight infections.
Rolling and adhesion can be simulated with flow assays. In a rolling and adhesion assay, leukocytes are perfused over a surface with a protein coating or a cell layer. Their adhesion to and their interaction with the surface can be analyzed under various conditions (e.g., after gene knockdown or drug treatment).
Murphy K, Weaver C, Mowat A, Berg L, Chaplin D, Janeway CA. Janeway's Immunobiology. Garland Science; 2017.
Find Out MorePlease find more detailed information about the planning, conduction, and data analysis of cell culture under flow assays in our Application Chapter. |
|
ibidi Solutions for Shear Stress Assays
The ibidi Pump System is ideal for long-term cell culture under flow with defined shear stress values and is compatible with all µ-Slides with Luer adapters. It simulates defined continuous and pulsatile laminar flow, and oscillatory flow to study cells in a more physiological environment. It is optimal for rolling and adhesion assays, transmigration, and invasion studies. Also, cells, spheroids, and organoids can be perfused for optimal nutrition. |
|
ibidi provides a variety of Channel Slides with different geometries. The µ-Slide I Luer family have one channel for standard flow experiments and rolling and adhesion assays. The µ-Slide VI has 6 channels and can be used for parallel flow assays. Both are available with the ibidi Polymer Coverslip and the ibidi Glass Coverslip, plus different heights and coatings. |
|
In the µ-Slide III 3D Perfusion, single cells, spheroids, or organoids can be cultivated in or on a gel layer or embedded in a 3D matrix. The special channel geometry allows for superfusion with a low flow rate, ensuring optimal oxygen and nutrient supply. This setup makes long-term cultivation possible for up to several weeks. Additionally, the thin coverslip bottom allows for high-resolution imaging. |
|
The μ-Slide I Luer 3D allows for creating an endothelial barrier without the need of an artificial filter membrane. Endothelial cells can be seeded on a suitable gel matrix, such as Collagen Type I. After connecting the slide to a pump and applying defined shear stress, an in vivo-like endothelial barrier is created, which is useful for rolling and adhesion assays or transendothelial migration studies (e.g., of leukocytes). |
|
Endothelial cells can be seeded on a suitable gel matrix, such as Collagen Type I. |
|
Selected References
Rolling and adhesion assays of T cells and human brain microvascular endothelial cells (HBMEC) and human umbilical vein endothelial cells (HUVEC). Cells were cultured in the μ-Slide I 0.4 Luer and kept under flow conditions using the ibidi Pump System. Physiological conditions were maintained using an ibidi Stage Top Incubator.
Klinger M, Zugmaier G, Nägele V, et al. Adhesion of T Cells to Endothelial Cells Facilitates Blinatumomab-Associated Neurologic Adverse Events. Cancer Res. 2020;80(1):91-101. doi:10.1158/0008-5472.CAN-19-1131.
Read article
Migration of neutrophils under shear stress in the µ-Slide I 0.8 Luer
Lim K, Hyun YM, Lambert-Emo K, et al. Neutrophil trails guide influenza-specific CD8⁺ T cells in the airways. Science. 2015;349(6252):aaa4352. doi:10.1126/science.aaa4352.
Read article
Flow guidance analysis of primary human effector T lymphocytes crawling on substrates using the µ-Slide VI 0.4 and the ibidi Pump System
Hornung A, Sbarrato T, Garcia-Seyda N, et al. A Bistable Mechanism Mediated by Integrins Controls Mechanotaxis of Leukocytes. Biophys J. 2020;118(3):565-577. doi:10.1016/j.bpj.2019.12.013.
Read article
Read on and learn more about methods for Immune Cell Analysis, Tumor Microenvironment and Immunooncology, or Immune Cell Migration and Chemotaxis.