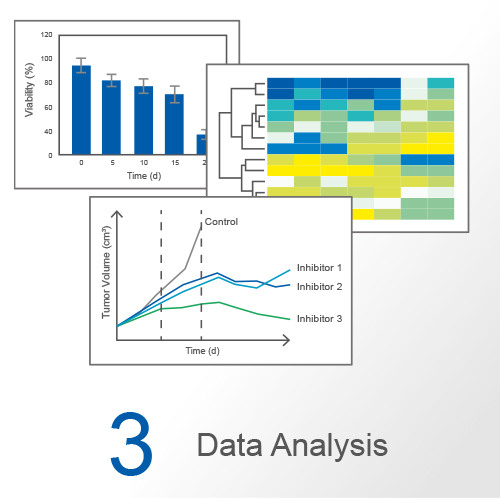
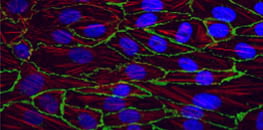
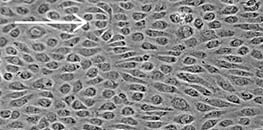
Cell Culture Under Flow
Culture Your Cells Under Physiological Conditions
Traditional static cell culture techniques fail to mimic the dynamic nature of in vivo environments, limiting the physiological relevance of the experimental results. ibidi provides advanced solutions for conducting and analyzing cell culture under flow assays under in vivo-like conditions. By integrating various ibidi products, researchers can effortlessly manage complex designs, enhancing the robustness and efficiency of their scientific investigations. With the ibidi Pump System, the µ-Slides, and the ibidi Stage Top Incubators, you can elevate your research to achieve more precise and reliable results.
This application chapter provides a detailed overview of the features and applications of the ibidi Pump System in combination with specialized channel slides. These slides are designed to stimulate the mechanical force generated by fluid flow, such as wall shear stress in blood and lymphatic vessels. They also support the long-term cultivation of both 2D and 3D cells by enhancing cellular viability and perforation through optimal nutrient supply and waste removal through dynamic cell perfusion.
Experimental Workflow of a Cell Culture Under Flow Assay
Systems and Applications for Cell Culture Under Flow Assays
Cell culture under flow experiments demand absolutely precise and reliable setups. The ibidi products, including the ibidi Pump System, the µ-Slides, and the ibidi Stage Top Incubators, are designed for performing and analyzing cell culture under flow assays under physiologic conditions. Thanks to their flexibility and modularity, these systems can be easily tailored to meet your experimental requirements and can be used for creating a defined flow profile and wall shear stress as well as perfusion through 3D culture chips and platforms.

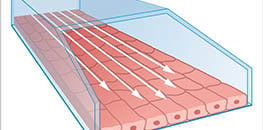
Simulation of Wall Shear Stress
Perfusion systems can simulate wall shear stress, which is a mechanical force that is created by fluid flow in biofluidic systems, including blood and lymphatic vessels and nephrons. These systems support the physiological long-term cultivation of vascular endothelial and epithelial cells for the analysis of relevant readouts, including cell morphology, behavior, and physiology.

Perfused Cell Cultures
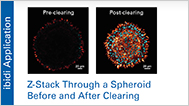
Perfusion of cell cultures using 3D culture chips and platforms enables physiological long-term 2D and 3D cell culture, and can be used for organoids, spheroids, and organs-on-a-chip. These systems facilitate continuous medium exchange, ensuring a constant supply of nutrients for optimal cell viability and health.

ibidi Solutions for Cell Culture Under Flow Assays
Find more products for cell culture under flow and cell perfusion.
ibidi Blog Articles |
Do you want to know "3 Reasons Why You Should Cultivate Endothelial Cells Under Flow"?
Discover how cell culture under flow is used to simulate endothelial dysfunction in our blog article "Shear Genius: Microfluidics in Atherosclerosis Research".
Scientific Posters
Presented at the International Cell Culture Under Flow Meeting 2024, Chicago, USA.
Presented at the SelectBio Conference Organoids and Spheroids Europe 2024, Rotterdam, The Netherlands.
Presented at the North American Vascular Biology Organization (NAVBO) Conference 2014, Monterey, California, USA.
Defining the Critical Shear Stress Range in Long Term HUVEC Cell Culture (PDF)
Presented at the 17th Barrier and Transporter Meeting 2015, Bad Herrenalb, Germany.
Selected Publications for Cell Culture Under Flow
The effect of different blood flow patterns on the endothelial lipidome was investigated using the ibidi Pump System and µ-Slides Luer.
Hong SG, Kennelly JP, Williams KJ, Bensinger SJ, Mack JJ. Flow-mediated modulation of the endothelial cell lipidome. Front Physiol. 2024;15:1431847. doi:10.3389/fphys.2024.1431847.
Read article
Endothelial cells were cultured under high laminar shear stress using the ibidi Pump System, µ-Slide 0.4 Luer, µ-Slide y-shaped, and µ-Slides With Test µ-Patterns RGD, to study how it influences inflammation.
Hong SG, Ashby JW, Kennelly JP, et al. Mechanosensitive membrane domains regulate calcium entry in arterial endothelial cells to protect against inflammation. J Clin Invest. 2024;134(13):e175057. doi:10.1172/JCI175057.
Read article
The ibidi Pump System was used to develop an in vitro bypass flow model to simulate disturbed flow and other hemodynamics patterns.
Xiao Z, Postma RJ, van Zonneveld AJ, et al. A bypass flow model to study endothelial cell mechanotransduction across diverse flow environments. Mater Today Bio. 2024;27:101121. doi:10.1016/j.mtbio.2024.101121.
Read article
Shear stress-modulated chromatin accessibility: This protocol demonstrates culture and harvesting of endothelial cells for ATAC-seq, highlighting growth factor stimulation and different shear stress rates.
Jatzlau J, Mendez PL, Altay A, et al. Fluid shear stress-modulated chromatin accessibility reveals the mechano-dependency of endothelial SMAD1/5-mediated gene transcription. iScience. 2023;26(9):107405. doi:10.1016/j.isci.2023.107405.
Read article
The ibidi Pump System and µ-Slides Luer were used to study how different shear stress patterns influence Mitochondrial Ca2+ Uniporter (MCU) activity in vascular endothelial cells.
Patel A, Pietromicca JG, Venkatesan M, et al. Modulation of the mitochondrial Ca2+ uniporter complex subunit expression by different shear stress patterns in vascular endothelial cells. Physiol Rep. 2023;11(3):e15588. doi:10.14814/phy2.15588.
Read article
Dynamic endothelial cell cultivation on scaffolds using the ibidi Pump System and µ-Slide I Luer 3D.
Loewner S, Heene S, Cholewa F, Heymann H, Blume H, Blume C. Successful endothelial monolayer formation on melt electrowritten scaffolds under dynamic conditions to mimic tunica intima. Int J Bioprint. 2024;10(1):1111. doi: 10.36922/ijb.1111.
Read article
The ibidi Pump System and µ-Slides I 0.8 Luer were used do simulate laminar and disturbed flow in lymphatic endothelial cells to analyze the function of FOXC2 in lymphatic vasculature.
Sabine A, Bovay E, Demir CS, et al. FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J Clin Invest. 2015;125(10):3861-3877. doi:10.1172/JCI80454.
Read article
User Protocols for Cell Culture Under Flow
Application Notes for Cell Culture Under Flow
Webinars for Cell Culture Under Flow
Cell Culture Under Flow Videos
Frequently Asked Questions
What is cell culture under flow and why is it used?
Cell culture under flow refers to growing adherent cells while applying controlled fluid flow instead of using static conditions. This introduces shear stress, a key mechanical signal experienced by many cells in vivo. Culturing under flow supports more physiological cell behavior, such as alignment, gene regulation, and enhanced barrier formation, making results more biologically relevant than in static conditions.
Which cell types benefit most from flow-based culture systems?
Cell types that are naturally exposed to fluid flow, such as vascular or lymphatic endothelial cells and epithelial cells from the lung or kidney, benefit most. Applying physiological shear stress in vitro helps these cells develop correct morphology and function, better reflecting their physiological state.
What kinds of flow can be simulated and how do they differ?
Using the ibidi Pump System and compatible Channel Slides, you can simulate unidirectional, pulsatile, or oscillatory flow. Unidirectional flow represents steady conditions like in small, healthy blood vessels and supports cell alignment and homeostasis. Pulsatile flow mimics heartbeat-driven rhythms with alternating shear phases, common in larger arteries. Oscillatory flow reverses direction and models disturbed or pathological states, such as those near vessel branches. These controlled profiles help mimic diverse in vivo flow environments more accurately.
How long can cells be cultured under flow conditions?
With the ibidi Pump System, cells can be cultured under continuous flow for extended periods, from several hours to multiple days or weeks. The system supports long-term perfusion experiments, making it ideal for studies on chronic stimulation, cell differentiation, or sustained viability in dynamic environments.
How does flow affect cell behavior compared to static culture?
Flow conditions, especially shear stress, induce major changes in cell shape, alignment, cytoskeletal organization, and gene expression. Compared to static culture, cells grown under flow exhibit more in vivo–like behavior, especially in terms of morphology and barrier function.
Can flow experiments be combined with immunofluorescence or live imaging?
Yes. Cells can be cultured under flow, then fixed directly within the Channel Slides for immunofluorescence staining or other endpoint assays. Alternatively, you can also monitor cells using live imaging in combination with Stage Top Incubator during the experiment. This enables dynamic observation of cellular responses while applying defined shear stress.
Which parameters are important when setting up a flow experiment?
Key parameters include shear stress, flow rate, medium viscosity, and channel geometry. These determine the mechanical environment cells experience. Accurate control of these parameters is critical for reproducible and physiologically relevant experiments. For detailed guidance, refer to our application notes under "Culture of Cell Monolayers Under Flow."
List of pages in %s: