ibidi Paper Award 2022:
Cancer Research
ibidi is searching for Early Career Scientists who have made outstanding contributions in the field of cancer research. Three top candidates have now been selected by an external jury and will be awarded the ibidi Paper Award 2022 for their contributions to this field, and will receive 500€ each.
Congratulations to the winners!

Thank you for submitting your publication to our 2022 ibidi Paper Award and making it a great success.
A special thank you to Dr. Maria Oliveira, Prof. Dr. Heinrich Leonhardt, and Prof. Dr. Antonio García de Herreros Madueño for their support in the winner selection process as external jury members.
Winners

Lena Neufeld, M.Sc.
Tel Aviv University, Israel

Claudio Ballabio, PhD
Francis Crick Institute, UK

Soma Ghosh, PhD
MD Anderson Cancer Center, USA
Lena Neufeld for her first author publication in Science Advances:
Microengineered perfusable 3D-bioprinted glioblastoma model for in vivo mimicry of tumor microenvironment
Lena Neufeld, M.Sc. Tel Aviv University, Tel Aviv, Israel
Lena is a highly experienced Bio-Chemical Engineer and a passionate Biomaterial Scientist with over 10 years of multidisciplinary research, specializing in a wide range of technological fields such as Drug Delivery Systems, Tissue Engineering, Cancer Biology, and Microfluidics. Lena holds a B.Sc. and M.Sc. (Cum Laude) in Chemical Engineering from the Technion, Israel Institute of Technology. She is finishing her Ph.D., focusing on 3D-Bioprinting Cancer Models and Polymers Science in the lab of Prof. Satchi-Fainaro at Tel Aviv University, the Sackler Faculty of Medicine. Her innovative research yielded not only patents and publications in high-impact scientific journals such as 'Science Advances', but also a start-up company for Personalized Medicine is about to be established based on her research.

Why Lena Neufeld's first author publication convinced the jury:
This study recapitulated the tumor heterogenic microenvironment by creating fibrin glioblastoma bioink consisting of patient-derived glioblastoma cells, astrocytes, and microglia. In addition, Perfusable blood vessels were created using a sacrificial bioink coated with brain pericytes and endothelial cells. The authors could show that the response to pharmacological agents in these 3D-bioink platforms at the transcriptional level was very similar compared to orthotopic cancer mouse models, as opposed to 2D cultures on rigid plastic plates.
This publication convinced the jury because of its novelty, complexity, and validation using murine and human cells, and the transcriptional analysis performed thereafter.
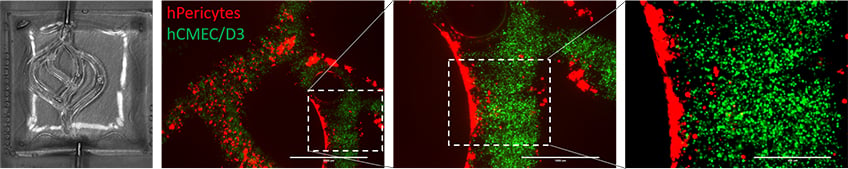
3D-bioprinted blood vessels inside cancer-fibrin bioink. The blood vessels are covered with human endothelial cells (green) and human microvascular pericytes (red)
Neufeld L, Yeini E, Reisman N, Shtilerman Y, Ben-Shushan D, Pozzi S, Madi A, Tiram G, Eldar-Boock A, Ferber S, Grossman R, Ram Z, Satchi-Fainaro R. Microengineered perfusable 3D-bioprinted glioblastoma model for in vivo mimicry of tumor microenvironment. Sci Adv. 2021 Aug 18;7(34):eabi9119. doi: 10.1126/sciadv.abi9119.
Read article
Claudio Ballabio for his first author publication in Science Advances:
Notch1 switches progenitor competence in inducing medulloblastoma
Claudio Ballabio, PhD, The Francis Crick Institute, London, UK
Claudio received his Master's degree in Molecular and Cellular Biotechnology at the University of Trento (Italy). In 2016, he joined the Armenise-Harvard Laboratory of Brain Disorders and Cancer at the University of Trento in the Department of Cellular, Computational and Integrative Biology as a Ph.D. student. Under the guidance of Professor Luca Tiberi, he studied Group 3 medulloblastoma, an aggressive pediatric brain tumor. He developed novel tools to model this tumor using mice and human pluripotent stem cell-derived cerebellar organoids. He then used these models to identify Group 3 medulloblastoma cell of origin, combining lineage tracing, single-cell transcriptome analyses, and live organoid imaging. Claudio was awarded his PhD in 2020. He is now a postdoctoral fellow in the Cancer Neuroscience laboratory at the Francis Crick Institute (London), investigating peripheral neurons' role in the tumor microenvironment.

Why Claudio Ballabio's first author publication convinced the jury:
In this publication, the authors investigate the role of the Notch 1 pathway in the initiation of Group 3 medulloblastoma (MB), a malignant childhood brain cancer with a poor prognosis.
They have established an elaborated mouse model together with single-cell transcriptome analyses, and studies on organoids. In addition, this study is of significant relevance, because it is directly related to tumor diseases in children.
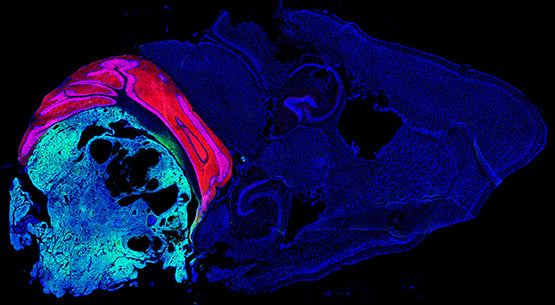
Section of a mouse brain with Group 3 medulloblastoma, expressing tdTomato in cerebellar granule neurons (red) and Venus in cancer cells (green)
Ballabio C, Gianesello M, Lago C, Okonechnikov K, Anderle M, Aiello G, Antonica F, Zhang T, Gianno F, Giangaspero F, Hassan BA, Pfister SM, Tiberi L. Notch1 switches progenitor competence in inducing medulloblastoma. Sci Adv. 2021 Jun 23;7(26):eabd2781. doi: 10.1126/sciadv.abd2781.
Read article
Soma Ghosh for her first author publication in Cell Reports:
PD-L1 recruits phospholipase C and enhances tumorigenicity of lung tumors harboring mutant forms of EGFR
Soma Ghosh, PhD, The MD Anderson Cancer Center, Houston, Texas, USA
Soma has been in the field of cancer biology research for more than a decade. She completed her PhD in Cancer Biology from DRDO, INMAS, New Delhi, with a focus on low radiation in cancer. Following her PhD, she completed 2 years of industry research & analysis on oncology drugs and pipeline and her first post-doctoral research at the Weizmann Institute of Science in Israel, where she worked on understanding the regulation of PD-L1/ CD274 in EGFR mutated lung cancer cell lines. To date, she has authored and co-authored 15+ publications in peer-reviewed journals. She is passionate about the biology of tumor microenvironment and the avenues of targeted and combination therapies to increase patient longevity. She has garnered special interest in research around signaling networks on malignant transformation and progression of cancer cells. Continuing her passion for cancer research, she is currently pursuing her second post-doctoral fellowship at the MD Anderson Cancer Center in Houston, Texas, USA. Here her focus is on understanding new targets for HPV+ head & neck and cervical cancer patients to help identify novel treatment approaches that would have less toxicity and better efficacy than the current standard of care.

Why Soma Ghosh's first author publication convinced the jury:
Immunotherapy has become a revolution in cancer therapy. However, despite the great achievements of immune checkpoint blockers in several cancer types, many patients still present a lack of response. For instance, lung cancer patients with EGF Receptor (EGFR) mutations are resistant to immunotherapy with PD-1 blockers.
Ghosh et al. describe how high EGF signaling stabilizes PD-L1 RNA and enhances tumorigenesis and invasiveness. They also propose a novel molecular mechanism based on phospholipase C-g1 (PLCg1) recruitment to PD-L1 that enables EGF receptor phosphorylation and activation of PLCg1 that, in turn, through the stimulation of calcium flux, Rho GTPases, and protein kinase C, promotes an aggressive phenotype. In summary, Ghosh and colleagues demonstrate a novel role of PD-L1 as an essential factor in EGFR signaling and help to understand how this immunotherapeutic target works.
Ghosh S, Nataraj NB, Noronha A, Patkar S, Sekar A, Mukherjee S, Winograd-Katz S, Kramarsi L, Verma A, Lindzen M, Garcia DD, Green J, eisenberg G, Gil-Henn H, Basu A, Lender Y, WEiss S, Oren M, Lotem M, Geiger B, Ruppin E, Yarden Y. PD-L1 recruites phospholipase C and enhances tumorigenicity of lung Tumors harboring mutant forms of EGFR. Cell Rep. 2021 May 25;35(8):108181. doi: 10.1016/j.cerep.2021.109181.
External Jury "ibidi Paper Award 2022"
Maria José Oliveira, Ph.D., Group Leader
Maria J. Oliveira is the Coordinator of the Tumor and Microenvironment Interactions (TMI) Group and Principal Researcher at the i3S-Institute for Research and Innovation in Health (University of Porto). In 2004, she received her PhD in Health and Medical Sciences at Ghent University Hospital (Belgium), studying the role of bacteria on colorectal cancer invasion and dissecting the associated mechanisms. After a Postdoc at IPATIMUP (UPorto) working on gastric cancer invasion-associated signaling pathways, Maria joined the Institute for Biomedical Engineering (INEB, UPorto), where she initiated a new line of research. In January 2017, Maria established the TMI group, which focuses on studying the role of the tumor microenvironment, particularly that of immune cells, adipocytes, and extracellular matrix components, on the modulation of cancer cell invasion and metastasis. Maria's research is also dedicated to understanding how cancer cells escape immune surveillance and foreseeing the design of more efficient immunomodulatory therapies.

Heinrich Leonhardt, Ph.D., Professor
Heinrich Leonhardt is a Professor for Human Biology and Bioimaging at the Ludwig Maximilian University in Munich, Germany. He did his PhD at the Freie Universität and the Max Planck Institute for Molecular Genetics in Berlin. Then, as a postdoc at the Harvard Medical School in Boston, he studied the regulation of DNA methylation and functional nuclear architecture. In 1995, he returned to Berlin as a group leader at the Franz Volhard Clinic and the Max Delbrück Center for Molecular Medicine. Since 2002, he has been a Professor at Ludwig Maximilian University in Munich, focusing on the role and regulation of DNA modifications in development and disease, as well as the functional organization of mammalian nuclei. Throughout his career, he has been active in technology development (e.g., super-resolution and live-cell microscopy and nanobody and antibody technologies), and he has co-founded several companies.

Antonio García de Herreros Madueño, Ph.D., Professor
Antonio García de Herreros Madueño is a researcher and group leader at the Department of Medicine and Life Sciences for Cancer Research at the IMIM-Hospital del Mar, as well as a full Professor of Biochemistry and Molecular Biology at the Department of Experimental and Health Sciences at the Universitat Pompeu Fabra in Barcelona, Spain.
Antonio García de Herreros Madueño holds a degree in Chemistry, with a major in Biochemistry from the Universidad Complutense de Madrid, Spain. After performing his doctoral thesis at the Centro de Biología Molecular (Universidad Autónoma de Madrid), he completed his research training at Memorial Sloan-Kettering Cancer Center and Harvard Medical School. In 1991, he started his own research group at IMIM (Institut Hospital del Mar d'Investigaciones Mèdiques), Barcelona, Spain.
The main focus of his research is to understand Epithelial to Mesenchymal Transition (EMT), which is the process of cellular conversion of epithelial cells into fibroblast-like cells, which increases the invasiveness of tumor cells. In 2000, his research group published a seminal article characterizing the transcription factor Snail1 as a key repressor of E-cadherin expression and, therefore, as a key inducer of EMT.
Today, the research group of Antonio García de Herreros continues to investigate the role and function of Snail1 in tumor activation and progression, both in epithelial and tumor microenvironment cells. Moreover, the group focuses on identifying Snail1 inhibitors that could be used as potential antineoplastic agents.





