ibidi Paper Award 2023
Immunology Research
ibidi completed our search for Early Career Scientists who have made outstanding contributions to the field of immunology research. The top three candidates have now been selected by an external jury and will be awarded the ibidi Paper Award 2023 for their contributions to this field, and will receive 500€ each.
Congratulations to the winners!
Thank you for submitting your publication and making the 2023 ibidi Paper Award a great success.
A special thank you to Dr. Tim Lämmermann, Prof. Dr. Christian Münz, and Prof. Dr. Michael Dustin, the external jury members, for their support in the winner selection process.

Winners
Max Grönloh for his first author publication in EMBO Reports:
Endothelial transmigration hotspots limit vascular leakage through heterogeneous expression of ICAM-1

Max Grönloh, M.Sc.
Max is a cell biologist, specifically in the field of immunology and endothelial biology. He obtained his bachelor’s and master’s degrees (Cum Laude) at the University of Amsterdam, focusing on using advanced microscopy techniques to answer questions in cell biology. During his first master’s internship, he developed novel biosensors for MAP kinases and used them to study cellular heterogeneity in responses downstream of the Calcium-sensing receptor. In his second internship, he used super-resolution microscopy to study integrin activation at filopodia tips by motor protein Myosin10. Currently, Max is in the final year of his PhD studies, where he is researching leukocyte extravasation. He is mainly focused on figuring out what factors decide why leukocytes extravasates at specific non-random spots in the vasculature. Besides his PhD work, he is passionate about cell migration in general, cytoskeletal functions, and the newest developments in microscopy. He is now searching for opportunities after the PhD.
Why Max Grönloh’s first author publication convinced the jury:
Grönloh et al. discovered that endothelial transmigration hotspots limit vascular leakage during inflammation-induced extravasation. These hotspots are regions where leukocytes cross the endothelial monolayer during inflammation. The heterogeneous distribution of intercellular adhesion molecule-1 (ICAM-1) determines the location of the transmigration hotspot, while the loss of ICAM-1 heterogeneity results in the loss of transmigration hotspots but not necessarily in reduced transendothelial migration (TEM) events. The loss of endothelial hotspots results in increased vascular leakage during TEM.
This comprehensive study provides valuable insights into why some ICAM-1 targeted therapies have complicated effects, as they might even increase inflammation associated vascular leakage, something they would also be hoping to reduce with the therapy.

Image: the µ-Slide VI 0.4 was used to perfuse isolated primary human neutrophils over TNFa-inflamed HUVECs under physiological flow conditions. Video: Neutrophils are transmigrating through an endothelial monolayer. The neutrophils seem to transmigrate close to each other in a non-random manner, called transmigration hotspots.
Grönloh MLB, Arts JJG, Palacios Martínez S, van der Veen AA, Kempers L, van Steen ACI, Roelofs JJTH, Nolte MA, Goedhart J, van Buul JD. Endothelial transmigration hotspots limit vascular leakage through heterogeneous expression of ICAM-1. EMBO Rep. 2023 Jan 9;24(1):e55483. doi: 10.15252/embr.202255483.
Read article
Luise Appeltshauser for her first author publication in BRAIN:
Anti-pan-neurofascin antibodies induce subclass-related complement activation and nodo-paranodal damage

Luise Appeltshauser, Dr. med. M.Sc.
Luise is a resident in neurology and a postdoctoral researcher at the University Hospital of Würzburg, Germany. She finished her medical doctoral thesis in the group of Prof. Claudia Sommer and Dr. Kathrin Doppler, University of Würzburg “summa cum laude”, where she discovered new autoantigens in autoimmune neuropathies. Furthermore, she completed a Master’s degree in Experimental Medicine at the University of Würzburg. As a clinician scientist, she has been working on the clinical profile and pathophysiology of antibody-mediated neurological disorders ever since. She has established new cell culture models, imaging techniques, and diagnostic arrays in her lab, which help her to identify and investigate new target antigens in autoimmune neuropathies. She holds publications in high-impact scientific journals, has won several awards, and has obtained different research scholarships from her university. Luise is fascinated by bench-to-bedside research and hopes that her research directly improves the treatment and quality of life of individuals with severe autoimmune neurological disorders.
Why Luise Appeltshauser’s first author publication convinced the jury:
Anti-pan-neurofascin antibodies are associated with a new subtype of nodo-paranodopathy, which is relevant because it is associated with high morbidity and mortality. The pathogenicity, including IgG subclass-associated mechanisms, has not been unraveled, nor been directly compared to anti-neurofascin-155 IgG4 related pathology. In this publication, Appeltshauser et al. investigated Pan-neurofascin antibody associated nodo-paranodopathy, detected Subclass IgG3 and associated it with complement binding and cytotoxic effects in vitro.
In this study, the authors have succeeded in functionally characterizing the putative disease-causing antibodies of a subset of nodo-paranodopathy. They have investigated Fc mediated and Fc independent mechanisms of these pan-neurofascin specific IgG3 antibodies using elegant cell culture systems.
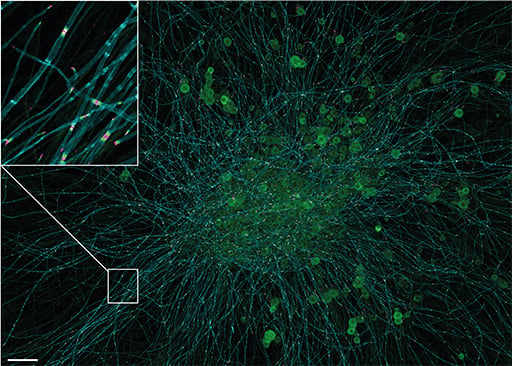
Confocal image of a murine dorsal root ganglion explant after 21 days of cultivation, with triple immunostaining for myelin markers (myelin associated glycoprotein, cyan) and the nodo-paranodal markers pan-neurofascin (magenta) and Caspr-1 (green). The myelinating co-culture model achieves mature nodes of Ranvier and compact myelin (inlet) and can be used to study myelination, node of Ranvier development, and pathogenic effects of human autoantibodies from patients with polyneuropathies. Scale bar = 100µm.
Appeltshauser L, Junghof H, Messinger J, Linke J, Haarmann A, Ayzenberg I, Baka P, Dorst J, Fisse AL, Grüter T, Hauschildt V, Jörk A, Leypoldt F, Mäurer M, Meinl E, Michels S, Motte J, Pitarokoili K, Stettner M, Villmann C, Weihrauch M, Welte GS, Zerr I, Heinze KG, Sommer C, Doppler K. Anti-pan-neurofascin antibodies induce subclass-related complement activation and nodo-paranodal damage. Brain. 2023 May 2;146(5):1932-1949. doi: 10.1093/brain/awac418.
Read article
Niklas Schmacke for his first author publication in Immunity:
IKKb primes inflammasome formation by recruiting NLRP3 to the trans-Golgi network

Niklas Schmacke, M.Sc.
Niklas received his Bachelor’s and Master‘s degrees from the University of Bonn, Germany. He joined Veit Hornung‘s lab at the Gene Center in Munich as a PhD student in 2017. Niklas’ research focuses on understanding how the immune system detects pathogens and other non-self entities. During his time in the lab, Niklas discovered a new priming modality of the NLRP3 inflammasome, a cell death and inflammation inducing protein complex that plays a critical role in diverse pro-inflammatory contexts, including infection and Alzheimer‘s disease. Additionally, he developed a technology for genome-wide screening of image-based cellular phenotypes using advanced microscopy. Currently, Niklas is working with Fabian Theis and Veit Hornung on the unbiased identification of phenotypes in single cell images using deep learning.
Why Niklas Schmacke's first author publication convinced the jury:
The NLRP3 inflammasome is a key activation pathway of the innate immune response and central to antimicrobial defense in mammalian species. Before the inflammasome is fully activated, an initial priming signal is required. Schmacke et al. make the surprising finding that human macrophages use another default pathway for NLRP3 priming than mouse macrophages. While mouse cells predominantly rely on NEK7 for the priming response, human myeloid cells circumvent NEK7 and instead use a previously unappreciated IKKβ-dependent pathway to prime NLRP3 by default. These novel findings improve our understanding of human inflammasome regulation, which is relevant to human disease and potential therapeutic interventions.
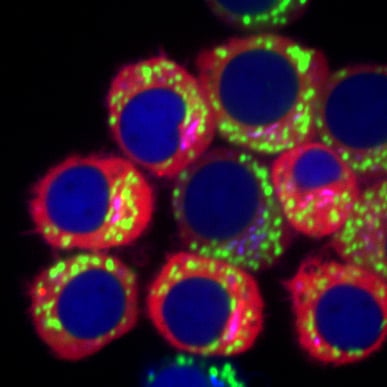
J774 mouse macrophages expressing a fusion of a fluorescent protein and the inflammasome sensor NLRP3 (red) as well as a trans-Golgi network marker (blue). The cells were stimulated with the pro-inflammatory bacterial component LPS and stained for mitochondria (green). The image was taken from live cells on a spinning disk confocal microscope at 100x magnification.
Schmacke NA, O'Duill F, Gaidt MM, Szymanska I, Kamper JM, Schmid-Burgk JL, Mädler SC, Mackens-Kiani T, Kozaki T, Chauhan D, Nagl D, Stafford CA, Harz H, Fröhlich AL, Pinci F, Ginhoux F, Beckmann R, Mann M, Leonhardt H, Hornung V. IKKβ primes inflammasome formation by recruiting NLRP3 to the trans-Golgi network. Immunity. 2022 Dec 13;55(12):2271-2284.e7. doi: 10.1016/j.immuni.2022.10.021.
Read article
External Jury "ibidi Paper Award 2023"

Tim Lämmermann, Ph.D., Group Leader
Dr. Tim Lämmermann is an independent junior group leader at the Max Planck Institute of Immunobiology and Epigenetics in Freiburg, Germany, where he heads the research group “Immune Cell Dynamics.” Tim studied Molecular Medicine in Erlangen, then moved to Munich to pursue his PhD thesis at the Max Planck Institute in Martinsried. Afterwards, he did his postdoctoral fellowship at the National Institutes of Health in Bethesda, USA. Since 2015, he has been a Max Planck Research Group Leader in Freiburg, where his lab investigates the basic cell biological mechanisms that shape single cell and population dynamics of immune cells in the complexity of inflamed and infected tissues. By using a broad range of microscopy and imaging techniques, his team explores the strategies that cells of the innate immune response have developed in order to move individually, or in a collective with other cells, to achieve an optimal immune response.

Christian Münz, Ph.D., Professor
Prof. Christian Münz is professor and co-director of the Institute of Experimental Immunology at the University of Zürich in Switzerland. He received his Ph.D. in 1998 from the German Cancer Research Center in Heidelberg and the Institute for Cell Biology at the University of Tübingen. In 1998, he joined The Rockefeller University as a postdoctoral fellow in the Laboratory of Cellular Physiology and Immunology. At the same institution, he became research assistant professor in 2001, and assistant professor and head of the Laboratory of Viral Immunobiology in 2003. In 2008, he returned to Europe to the University of Zürich and became professor and co-director of the Institute of Experimental Immunology. His laboratory studies the immune control of the human persistent and oncogenic Epstein Barr and Kaposi sarcoma-associated viruses, focusing on dendritic cells, natural killer cells, and T cells, as well as antigen processing by autophagy. He has published more than 300 articles on these and related topics, and has received both the Burroughs Welcome Fund Investigators in Pathogenesis of Infectious Disease Award and the Sobek Award, among other honors for his research.

Michael Dustin, Ph.D., Professor
Prof. Michael Dustin is the Kennedy Trust Professor of Molecular Immunology at the University of Oxford and Director of Research of the Kennedy Institute of Rheumatology. He has a B.A. in Biology from Boston University (1984) and a Ph.D. in Cell and Developmental Biology from Harvard University (1990). His interests are cell biology and immunology. His lab at Washington University led pioneering work on the dynamics of the immunological synapse in the 1990s. His work at the NYU Grossman School of Medicine in the 2000s explored in vivo dynamics of the immune response using two-photon laser scanning microscopy, and continued work on the dynamics of the immunological synapse formation using supported lipid bilayers. He moved his lab to the University of Oxford in 2013, with the support of a Principal Research Fellowship from Wellcome and the Kennedy Trust for Rheumatology Research. His work over the last eight years—generously supported by the ERC—has explored the nanoscale organization of the immunological synapse leading to a basic description of the supramolecular assemblies that make up the mature immunological synapse. Some surprising findings have included synaptic ectosomes involved in T cell help and supramolecular attack particles that mediate cytotoxicity, both of which are found in the center of the immunological synapse. Prof. Dustin received a Presidential Early Career Award in Science and Engineering in 2000 and is an elected member of EMBO and the National Academy of Sciences, USA.
Check out the winners of the ibidi Paper Award 2022 (Cancer Research) here.




