In our body, endothelial cells are surrounded by the basement membrane, which is a thin and highly specialized extracellular matrix (ECM). When endothelial cells, such as human umbilical vein cells (HUVECs), are seeded onto a basement membrane-like surface (e.g., Matrigel®) they form capillary-like structures in vitro, which recapitulates angiogenesis.
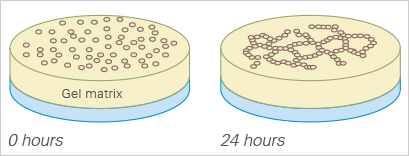
This so-called tube formation assay has been widely applied to solve a variety of experimental questions, such as:
- What is the pro- or anti-angiogenic potential of a specific drug?
- Which genes and pathways are involved in angiogenesis?
- What is the effect of inhibitors or enhancers on tube formation?
- How are signals transduced during angiogenesis?
- What are the cytoskeletal effects during angiogenesis?
- Which cells are endothelial progenitors?
Live cell imaging using the ibidi Stage Top Incubation System shows the tube formation of HUVECs in a tube formation assay.
The basement membrane surface and its components are the key to a successful tube formation assay. Different types of basement membranes can be applied (e.g., Matrigel®, collagen, or other hydrogel matrices), which can result in different tube formation rates. Endothelial cells differentiate morphologically to form tubes. The cells first attach to the matrix and migrate towards each other. Then, they attach to other cells, align, and form capillary-like tubes. The tubes maturate until the cells finally undergo apoptosis, resulting in detachment from the surface and tube destruction.
A tube formation assay is performed by first seeding single cells, and then observing and imaging the tube formation over time. Several readouts can be imaged and analyzed over time, such as tube length and the number of loops. Typically, tubes are formed within a few hours, making the tube formation assay a rapid tool for angiogenesis quantification in the research fields of embryonic development, cancer, wound healing, and tissue repair.
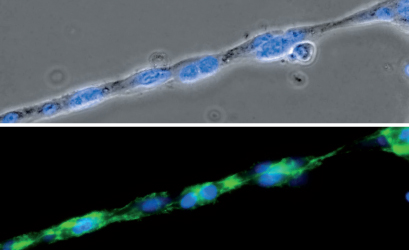
Phase contrast (top) and fluorescence microscopy (bottom) of a single strand composed of HUVEC cells during a tube formation assay in the µ-Slide 15 Well 3D. The F-actin cytoskeleton is stained green and the cell nuclei are stained blue.
Tube formation assays are a widely used in vitro tool for accessing angiogenesis in an easy, cost-effective, and reproducible fashion. The use of only one cell type guarantees well-defined experimental parameters, which strongly facilitates assay analysis. Using the ibidi µ-Slide 15 Well 3D or the μ-Plate 96 Well 3D for tube formation assays allows for live cell microscopy with all cells in focus on one 2D cell layer.
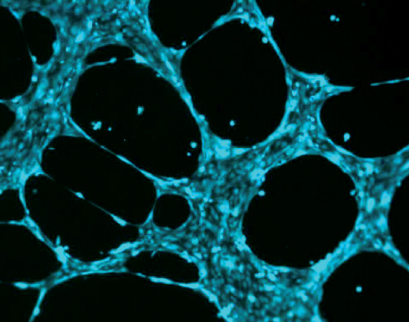
Characteristic pattern of human endothelial cells (HUVEC) stained with Calcein.
First tube formation assay:
Kubota Y, Kleinman HK, Martin GR, Lawley TJ (1988) Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol 107(4):1589–98.
Read article
Review about angiogenesis assays:
Arnaoutova I, Jay G, Kleinman HK, Benton G (2009) The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis 12:267–274. 10.1007/s10456-009-9146-4.
Read article
Tube formation assay protocol:
Arnaoutova I, Kleinman HK (2010) In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc 5(4):628–635. 10.1038/nprot.2010.6.
Read article
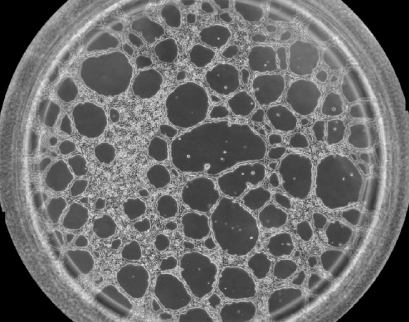
Phase contrast image showing one well of the µ-Slide 15 Well 3D with HUVEC cells on Matrigel® after 12 hours of incubation during a tube formation assay.
In our Application Notes and video, you will find detailed information on the setup, optimization, data analysis, and interpretation of tube formation assays:
- AN 05: Tube Formation in µ-Plate 96 Well 3D (PDF)
- AN 19: Tube Formation (PDF)
- AN 27: Optimizing Tube Formation Assays (PDF)
- AN 66: Tube Formation Assay With Laminin-Collagen I Gel in the μ-Slide 15 Well 3D (PDF)
- AN 70: Data Analysis of Tube Formation Assays (PDF)
- MV 32: Tube Formation Assays Using the µ-Slide 15 Well 3D
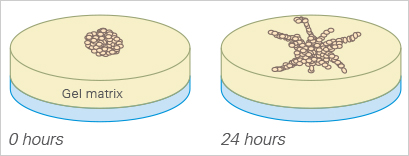
Similar to the tube formation assay, the sprouting assay can also be used to quantify angiogenesis in vitro.
Here, either multicellular spheroids or pieces of tissue (e.g., from the aorta) are placed into or onto a gel matrix (e.g., Matrigel®, collagen), where they then form sprouts. For analysis, the sprouting of the cell clusters is imaged at defined time points and the sprout length is measured. The sprouting assay, like the tube formation assay, needs a well-defined thickness of the gel layer, which is standardized when using the µ-Slide 15 Well 3D and the μ-Plate 96 Well 3D.
Stahl A, Wu X, Wenger A, Klagsbrun M, Kurschat P (2005) Endothelial progenitor cell sprouting in spheroid cultures is resistant to inhibition by osteoblasts: A model for bone replacement grafts. FEBS Lett 579(24):5338–5342. 10.1016/J.FEBSLET.2005.09.005.
Read article
Additional Angiogenesis Assays
Some additional in vitro angiogenicapproaches are the rat/mouse aortic ring assay and the chick aortic arch assay. With these assays, aortic rings from either rats or mice, or aortic arches from chick embryos are cultivated on a gel matrix (e.g., collagen or Matrigel®). Subsequently, the outgrowth of endothelial cells from these explants is monitored and quantified.
In vivo, angiogenesis can be studied in fertilized chick eggs with the chorioallantoic membrane (CAM) assay. A hole is cut into the shell of a developing chick egg, and the compound or graft of interest is placed on the CAM. The egg is incubated for several days and then the CAM angiogenesis is quantified.
In the corneal angiogenesis assay, vessel growth is induced in vivo in the normally avascular cornea of the rabbit or the mouse eye. After several days, the area of corneal neovascularization is assessed for quantification.
In the in vivo Matrigel® plug assay, a mixture of Matrigel® and cells, or pro-/antiangiogenic substances, is implanted subcutaneously into mice. The blood vessels that have entered the plug are quantified.
Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N (2003) Angiogenesis assays: a critical overview. Clin Chem 49(1):32–40. 10.1373/49.1.32.
Read article
Masson V, et al. (2002) Mouse Aortic Ring Assay: A New Approach of the Molecular Genetics of Angiogenesis. Biol Proced Online 4:24–31. 10.1251/bpo30.
Read article
Moreno-Jiménez I, et al. (2016) The chorioallantoic membrane (CAM) assay for the study of human bone regeneration: a refinement animal model for tissue engineering. Sci Rep 6(1):32168. 10.1038/srep32168.
Read article
Rogers MS, Birsner AE, D’Amato RJ (2007) The mouse cornea micropocket angiogenesis assay. Nat Protoc 2(10):2545–2550. 10.1038/nprot.2007.368.
Read article
Read on and learn more about Angiogenesis in Development and Disease or the Experimental Workflow of a tube formation assay.




