3D Cell Culture: A Definition
Most cells in living tissue grow in a three-dimensional architecture, where they communicate and interact with each other and their surroundings. Animal cells are embedded in the extracellular matrix (ECM), which is composed of proteoglycans and fibrous proteins (mainly collagen, elastin, and fibronectin). This complex, dynamic, and tissue-specific 3D structure provides physical scaffolding for the cells and initiates cues that influence cell differentiation and behavior.
When cultured in a traditional, two-dimensional in vitro environment, the cells are attached to a flat surface (e.g., a monolayer in a standard cell culture dish) and can only grow and migrate on the substrate. In a 3D in vitro setup, the cells are grown in suspension on a non-adhesive surface, or they can be embedded in or on a 3D matrix (e.g., Matrigel® or Collagen I) that mimics the ECM and allows them to grow in all three directions.
In many cases, a 3D environment reflects the in vivo situation more accurately. This should be considered when analyzing cell behavior, differentiation, response to drug treatment, and gene expression. It is therefore not surprising that many cell culture approaches have been adapted to a 3D setting. This includes drug screenings that use spheroids and organoids, which are indispensable nowadays as tumor models.
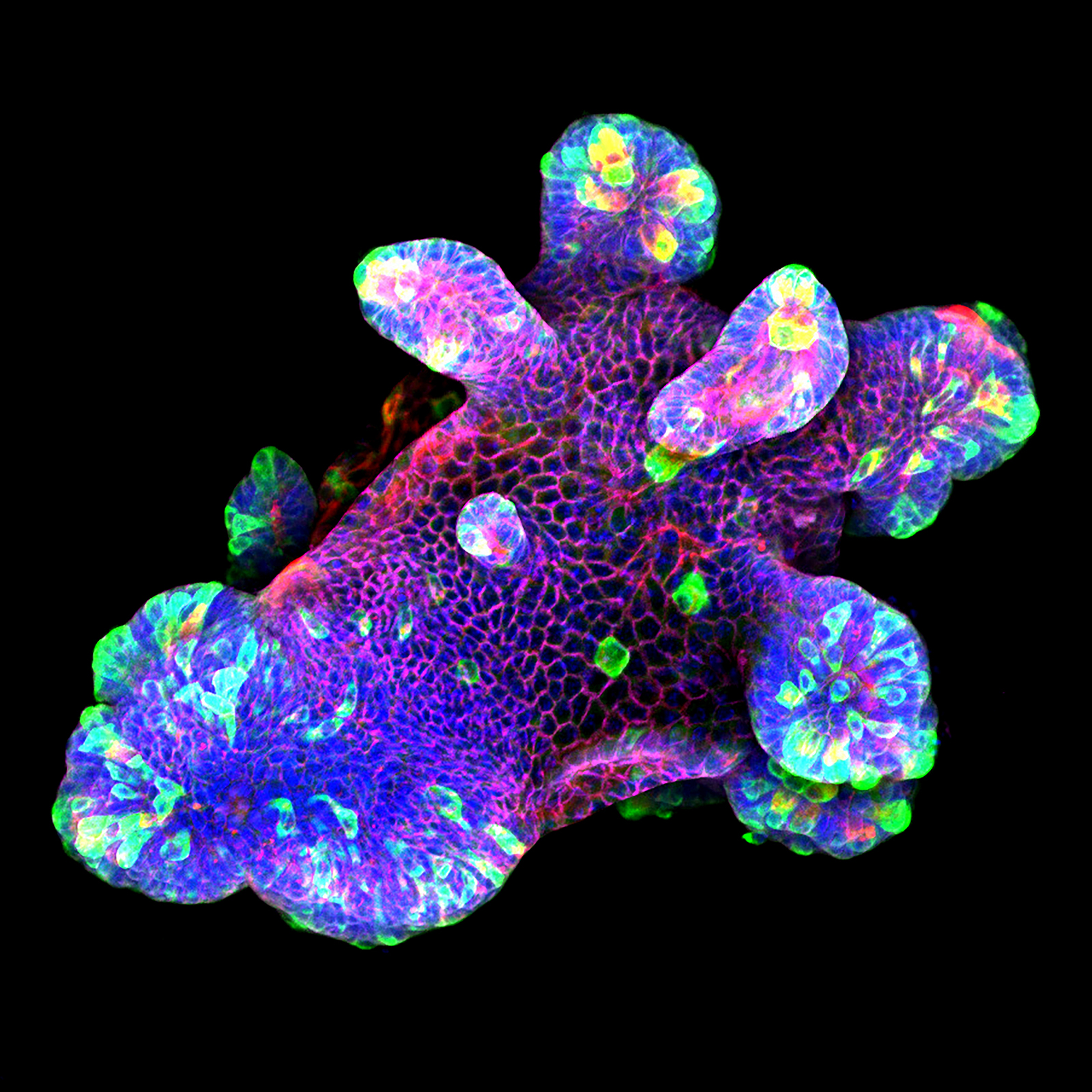
3D culture of an IL-22-treated mouse small intestine organoid grown in Matrigel® drops using an ibidi µ-Slide 8 Well. Cell image by Naveen Parmar, Norwegian University of Science and Technology (NTNU), Trondheim, Norway.
Although 3D cell culture-based experiments already offer a more in vivo-like approach when compared to 2D culture, multiple advancements to obtain even more realistic culture conditions have recently been established. This includes the dynamic flow culture of 3D cells, the simultaneous co-cultivation of multiple cell types in the same dish, and 3D printer-based bioprinting.
Edmondson R, Broglie JJ, Adcock AF, Yang L (2014) Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 12(4):207–18. 10.1089/adt.2014.573.
Read article
Frantz C, Stewart KM, Weaver VM (2010) The extracellular matrix at a glance. J Cell Sci 123(Pt 24):4195–200. 10.1242/jcs.023820.
Read article
Tuveson D, Clevers H (2019) Cancer modeling meets human organoid technology. Science 364(6444):952–955. 10.1126/science.aaw6985.
Read article
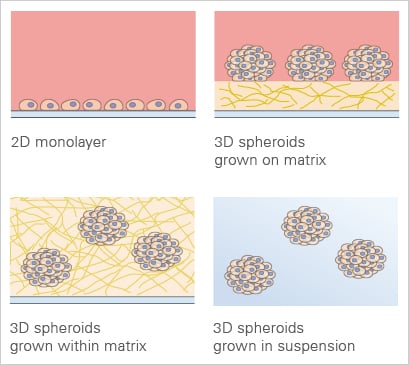
3D cell culture applications in comparison to the traditional 2D monolayer.
Scaffold-based 3D cell culture models provide a structural framework that mimics the extracellular matrix, supporting the growth and organization of cells in three dimensions. Unlike scaffold-free systems (which are self-forming aggregates without artificial supports, including spheroids and organoids), these models incorporate biocompatible materials—such as hydrogels, synthetic polymers, or natural fibers—that facilitate cell attachment, proliferation, and differentiation. This environment not only closely resembles in vivo conditions but also allows for the inclusion of multiple cell types, which can interact and develop complex tissue structures. Such models are crucial for studying cell interactions, tissue development, and disease progression, and are increasingly used in tissue engineering and regenerative medicine. Scaffold-based models enhance the physiological relevance of the studies, thereby improving the predictive accuracy of drug responses and biological behaviors.

Scaffold-based 3D culture of single cells in a matrix that mimics the physiologic environment.
Spheroids, clusters of cells forming under three-dimensional, non-adherent culture environments, are primarily composed of fully differentiated cells due to their usual lack of stem cells. This composition generally precludes them from self-renewal and further differentiation. However, tumor cell spheroids are an exception. Owing to the inherent unlimited proliferative capacity of cancer cells, these spheroids can divide and renew themselves, making them an invaluable tool for studying tumor cell behavior, especially in large-scale drug screenings. The creation of spheroids often employs techniques like the hanging drop method, which is a scaffold-free suspension approach. Spheroids offer important insights into cellular behaviors in a more in vivo-like environment, enhancing the relevance of findings in biomedical research.
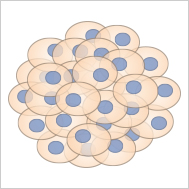
Spheroids are cell aggregates, which are often generated from cancer cells.
Organoids are cultured "mini organs" and can be generated from pluripotent stem cells (PSCs). When these cells are cultivated in a three-dimensional scaffold (e.g., Matrigel® or collagen), they differentiate into organ-specific cell types, undergoing important steps of organogenesis. Cultivation methods for organoids are becoming more and more sophisticated, making these 3D cell models increasingly accurate representations of real organs.
The first generation of intestinal organoids, created from an Lgr5+ stem cell by Sato et al., initiated many protocols for organoid generation from different organs, such as intestine, liver, brain, prostate, kidney, pancreas, lung, and thyroid. Importantly, organoids can be edited using technologies such as CRISPR, making them a powerful tool for studies about organogenesis, personalized medicine, and drug screening.
Several procedures for the generation and cultivation of 3D cells were established. While the generation of spheroids usually follows highly standardized protocols, the cultivation of organoids often requires a more careful revision of existing procedures.
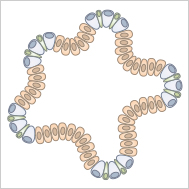
Organoids are cultured miniature versions of organs, which are derived from stem cells.
Sato T, et al. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459(7244):262–265. 10.1038/nature07935.
Read article
Drost J, Clevers H (2018) Organoids in cancer research. Nat Rev Cancer 18:407–418. 10.1038/s41568-018-0007-6.
Read article
Organ-on-a-chip (OOC) models are miniaturized, microfluidic cell culture systems that provide key structural and functional features of human tissues. By controlling fluid flow, applying physical stresses such as stretching or shear, and arranging cells in physiologically relevant layers, these systems can mimic organ functions far more realistically than traditional in-vitro cell cultures.
The first working OOC-model was developed in 2010 by researchers at the Wyss Institute. Their work described a "Lung-on-a-Chip" device to mimic lung alveolus, to simulate breathing, and to study lung responses to microparticles and inflammation.
Recent advances have expanded the field from single-organ models to interconnected multi-organ platforms capable of simulating whole-body interactions. Researchers are also integrating immune cells, microbiome components, and stem-cell-derived patient-specific tissues, creating chips that can model complex diseases, predict drug safety, and support personalized medicine. These technologies are now used for drug development, toxicity testing, and mechanobiology research, and they increasingly complement or replace certain animal studies.
Ingber, D.E. (2022) Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat Rev Genet 23, 467–491. 10.1038/s41576-022-00466-9.
Read article
Huh D, et al. (2010) Reconstituting organ-level lung functions on a chip. Science 25;328(5986):1662-8. 10.1126/science.1188302.
Read article
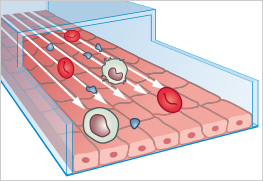
Organ-on-a-chip models are microengineered in vitro microfluidic devices that mimic the structure and function of human organs.
ibidi Blog Article |
Curious about how organoids are revolutionizing research? Then read the ibidi Blog article Organoids: The Miniature Labs to explore their power in disease modeling and drug discovery.
ibidi Tech Teaser
Find more organ-on-a-chip applications and use-cases here.
Experimental Examples
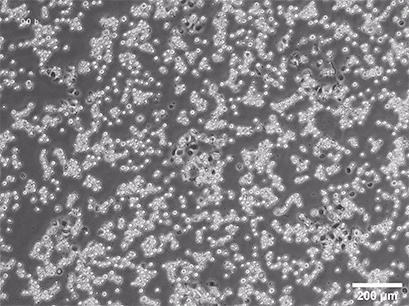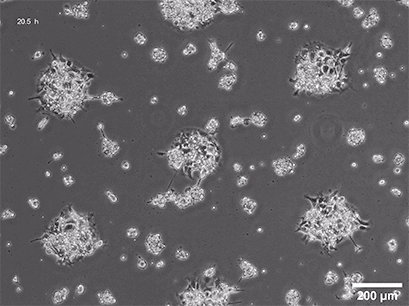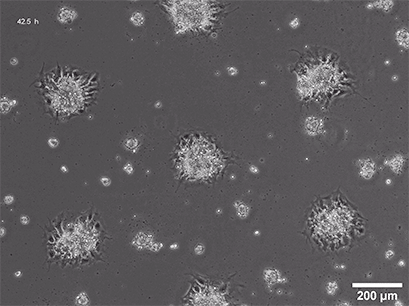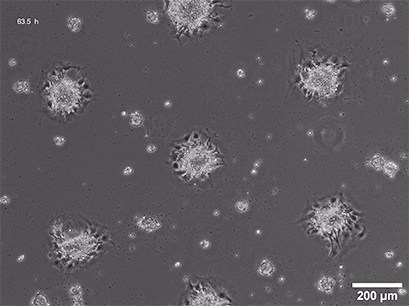
Spheroid formation of NIH-3T3 cells (murine embryo fibroblasts) on 200 µm adhesion spots using ibidi µ-Patterning. Spheroid generation was documented for 64 hours. Phase contrast live cell imaging, 4x objective lens.
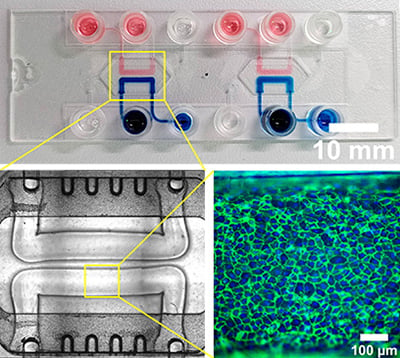
PTubeChip – 3D-printed organ models with perfused microchannels
Let Others Inspire You: Publications Using ibidi Products for 3D Cell Culture
hPSC-derived cardiac spheroids were embedded in Matrigel and plated into the sticky-Slide I Luer for cardiac contraction imaging.
Wang LT, Proulx MÈ, Kim AD, Lelarge V, McCaffrey L. (2021) A proximity proteomics screen in three-dimensional spheroid cultures identifies novel regulators of lumen formation. Sci Rep., 10.1038/s41598-021-02178-2.
Read article
The µ-Slide Spheroid Perfusion was chosen for the study of flow-induced changes to periodontal ligament stem cell spheroids.
Mishra A, Kai R, Atkuru S, Dai Y, Piccinini F, Preshaw PM, Sriram G (2023) Fluid flow-induced modulation of viability and osteodifferentiation of periodontal ligament stem cell spheroids-on-chip. Biomater Sci., 10.1039/d3bm01011b.
Read article
The µ-Plate 96 Well Square was used for the co-cultivation of hiPSC-derived high-grade glioma (HGG) organoids with forebrain organoids to study HGG infiltration into the brain.
Lago C, Gianesello M, Santomaso L, Leva G, Ballabio C, Anderle M, Antonica F, Tiberi L (2023) Medulloblastoma and high-grade glioma organoids for drug screening, lineage tracing, co-culture and in vivo assay. Nat Protoc., 10.1038/s41596-023-00839-2.
Read article
The µ-Slide 2 Well Co-Culture was used for the direct and indirect co-culture of differentiated SH-SY5Y cells and 3D Schwann cell-like cell spheroids.
Lin YJ, Lee YW, Chang CW, Huang CC (2020) 3D Spheroids of Umbilical Cord Blood MSC-Derived Schwann Cells Promote Peripheral Nerve Regeneration. Front Cell Dev Biol., 10.3389/fcell.2020.604946.
Read article
A pre-coated µ-Dish 35 mm, low Grid-500 was selected as a bioprinting support for a skin model used to study drug response.
Jeffries GDM, Xu S, Lobovkina T, Kirejev V, Tusseau F, Gyllensten C, Singh AK, Karila P, Moll L, Orwar O (2020) 3D micro-organisation printing of mammalian cells to generate biological tissues. Sci Rep., 10.1038/s41598-020-74191-w.
Read article
The µ-Plate 96 Well 3D was used for mono- and co-cultures of patient-derived pancreatic organoids with cancer-associated fibroblasts to analyze differential drug responses.
Schuth S, Le Blanc S, Krieger TG, Jabs J, Schenk M, Giese NA, Büchler MW, Eils R, Conrad C, Strobel O (2022) Patient-specific modeling of stroma-mediated chemoresistance of pancreatic cancer using a three-dimensional organoid-fibroblast co-culture system. J Exp Clin Cancer Res., 10.1186/s13046-022-02519-7.
Read article
The µ-Dish 35 mm, low Grid-500 was used for bioprinting a cancer model that successfully recapitulates the intercellular communication via the assembly of functional tunneling nanotube (TNT)-like cell projections.
Herrada-Manchón H, Celada L, Rodríguez-González D, Alejandro Fernández M, Aguilar E, Chiara MD (2021) Three-dimensional bioprinted cancer models: A powerful platform for investigating tunneling nanotube-like cell structures in complex microenvironments. Mater Sci Eng C Mater Biol Appl., 10.1016/j.msec.2021.112357.
Read article
The μ-Slide III 3D Perfusion and the ibidi Pump System were used to create a “cardiorenal-unit” in vitro model to study the cardiorenal axis.
Beatrice Gabbin, Viviana Meraviglia, Maricke L. Angenent, Dorien Ward-van Oostwaard, Wendy Sol, Christine L. Mummery, Ton J. Rabelink, Berend J. van Meer, Cathelijne W. van den Berg, Milena Bellin (2023) Heart and kidney organoids maintain organ-specific function in a microfluidic system. Materials Today Bio, 10.1016/j.mtbio.2023.100818.
Read article
Read on and learn more about A Typical 3D Cell Culture Workflow, the Generation and Cultivation of 3D Cell Models, discover methods of Advanced Cultivation of 3D Cell Models, or have a look at Experimental Examples.






