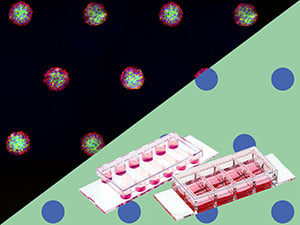
The ibidi µ-Patterning Technology
Achieve Precise Spatial Control Over Cell Adhesion With ibidi Micropatterning
The ibidi µ-Patterning technology enables spatially defined cell adhesion for various 2D and 3D cell culture applications.
Miniaturized adhesive patterns (e.g., squares or dots) are irreversibly printed on the non-adhesive Bioinert surface of the ibidi Polymer Coverslip, allowing for precisely controlled cell adhesion. The µ-Patterns are dry-stable, sterile, and ready to use.
The µ-Pattern, the Bioinert surface, and the ibidi Polymer Coverslip are all optimized for high resolution imaging and microscopy.
ibidi's Ready-to-Use µ-Patterning Products
Understanding the Key Elements of µ-Patterns
Every ibidi µ-Pattern is designed with precise spatial control, ensuring reproducible cell adhesion and organization. Let's break down the essential pattern components:
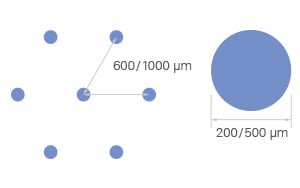
- Shape – Defines the geometry of the attachment area (e.g., cir = circle)
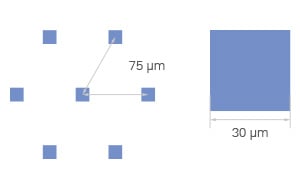
- Size – The diameter or width of the attachment area, measured in micrometers (µm) (e.g., 500 µm)
- Pitch – The spacing between adjacent shapes, measured from center to center (e.g., pit1000 = 1000 µm)
- Layout – The overall pattern arrangement, determining how attachment areas are structured (e.g., a hexagonal layout)
- Surface – The cell attachment surface where cells adhere, made of ibiTreat for optimal cell growth
Application Examples
Single-Cell Arrays
The size of the µ-Pattern can be adapted to the morphology of the cell type of interest, so that an array of single cells can be conveniently analyzed using applications such as high-resolution imaging.
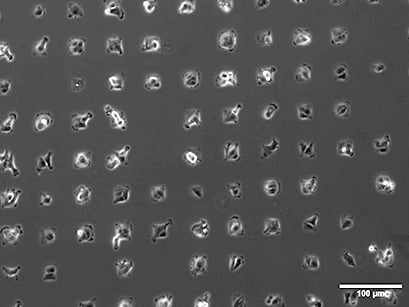
Single-cell array with RCC26 tumor cells. Spot size 40 µm x 40 µm. Phase contrast microscopy, 10x objective lens.
Spheroid Generation
Defined adhesion spots, surrounded by Bioinert, are able to catch all adherent single cells from a cell suspension. Bioinert is fully non-cell-attachable. This forces all cells to aggregate to each other at the adhesion spots, thus forming spheroids in a defined and controllable way.
Suspension of NIH-3T3 cell line seeded on 200 µm adhesion spots, 64 hours live cell imaging, phase contrast, 4x objective lens.
Interested in unlocking new possibilities with ibidi µ-Patterning?
Let's collaborate on a custom solution to meet your research needs! We will work with you to co-develop a tailor-made design here.

You need support or want to test the ibiTreat µ-Patterns first? Contact us at: info@ibidi.com or choose up to three free samples from our ibiTreat micropatterning standard products!
The size and the cell adhesion properties of the µ-Patterning can be adjusted to hold 3D spheroids and microtissues in position. Using this setup, cells in 3D can be studied during proliferation, differentiation, invasion, and migration.
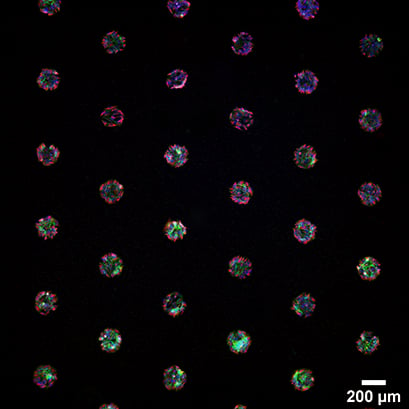
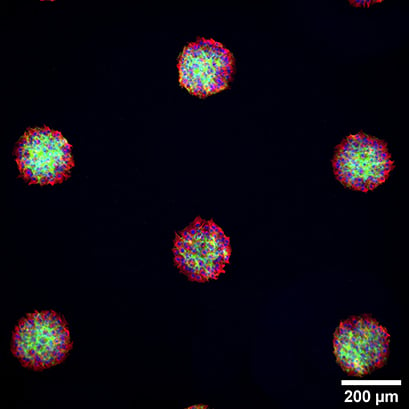
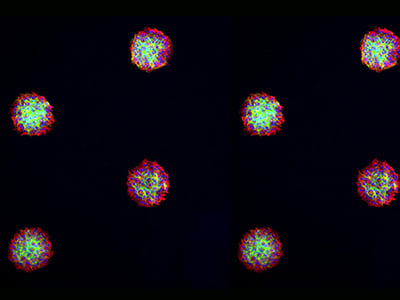
Immunofluorescence staining of spheroids from RCC26 (left) and Rat1 cells (right) in in the µ-Slide VI 0.4 Luer, patterned with 200 µm circles at 600 µm distance. The cells were stained with phalloidin (green) and alpha-tubulin (red). Nuclei were stained with DAPI (blue). Widefield fluorescence microscopy, 4x and 10x objective.
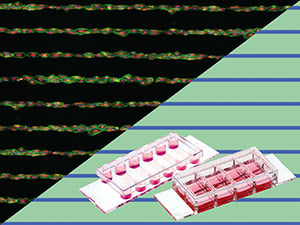
Line Arrays
The ready-to-use structured microlane environment supports e.g., neuronal cell and organelle outgrowth, including neurite extension and cell–cell communication. They provide a platform for studying geometry-driven cell migration and polarization, cytoskeletal dynamics, and organelle transport.
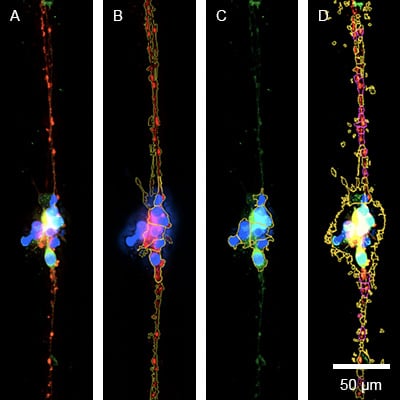
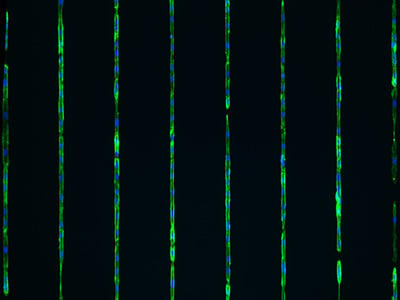
Neuronite outgrowth analysis. Fluorescence overlay image (A) of ß3 tubulin (red), VDAC (Mitochondria) (green/yellow), and the cell nuclei (DAPI). Visual segmentation is indicated by yellow lines to measure the ß3 tubulin staining (B), the VDAC signal in soma (C), and the VDAC signal in axons (indicated in pink) (D). Human iPSC derived sensory neurons were incubated on the μ-Slide VI 0.4 μ-Pattern ibiTreat, lin20, pit170 for 5 days and imaged on a Cytation 7 (Agilent) with a 40x objective lens. Image courtesy of Moran Amit's Lab, University of Texas.
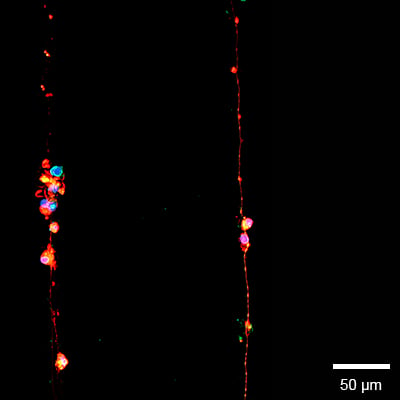
Voltage-dependent anion channel/mitochondrial porins (VDAC) and ß3-tubulin analysis to show the distribution of mitochondria along the cell. Fluorescence overlay image (A) of ß3-tubulin (red), VDAC (mitochondrial porins) (green/yellow) and the cell nuclei (DAPI). Human iPSC derived sensory neurons were incubated on the μ-Slide VI 0.4 μ-Pattern ibiTreat, lin20, pit170 for 5 days and imaged on a Cytation 7 (Agilent) with a 40x objective lens. Image courtesy of Moran Amit's Lab, University of Texas.
Integrating u-Patterning With the ibidi Pump System: Spheroid Culture Under Constant Media Circulation
By creating micropatterns within a µ-Slide VI 0.4 Luer, aggregates on the pattern can be constantly supplied with fresh media by perfusing the system with the ibidi Pump System.
By applying a constant media flux around the cell aggregates, spheroids become more compact and round.

3T3 fibroblasts were seeded on a patterned µ-Slide VI 0.4. A shear stress of 3 dyn/cm2 was applied 7 days after cell seeding. 15 h time lapse microscopy, 4x objective.
CAR-T Cell Killing Assays
CAR-T cells represent a promising new cancer therapy tool. Live cell imaging allows to analyze T cell/cancer cell interaction in real time with single cell resolution. However, analysis of confluent cell layers is very time-consuming and therefore not possible in high throughput screens. To facilitate high throughput label-free analysis of T cell potency in a live cell imaging setup, we generated arrays of homogenously distributed cancer cells. By combining optical analysis and advanced image processing, cytotoxic T cell activity over time on a single cell level can be evaluated without the use of any labeling.
Single Cells in a 2D Environment
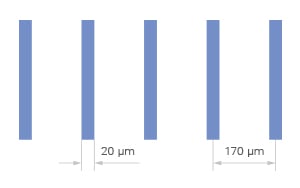
Time lapse microscopy of a CAR-T cell killing assay with RCC-26 tumor cells and JB4 T cells on a single cell pattern (µ-Slide VI 0.4, 20 µm squares, 120 µm distance, rectangular). Data were analyzed using FastTrack AI by MetaVi Labs.
Multi-Cell Spots in a 3D Collagen Matrix
RCC-26 cancer cells immobilized on multi-cell pads (µ-Slide VI 0.4, 200 µm circles, 600 µm distance, hexagonal). Effector T cells applied in a collagen I matrix (Collagen Type I, Rat Tail) induce apoptotic body formation of cancer cells.
Scientific Posters
Micropatterned Adhesion Sites for Spheroid Cultivation Under Flow (PDF)
Presented at the Annual Meeting of the Biophysical Society 2020, San Diego, USA.
Presented at the ASCB|EMBO Meeting 2019, Washington DC, USA.
Controlled Cell Adhesion With ibidi Micropatterning (PDF)
Presented at the µTAS Conference 2019, Basel, Switzerland.