Application Notes
Highlights
AN 08: Coating Protocols for ibidi Labware (PDF)
A detailed protocol on how to coat the ibidi labware (e.g., with Collagen, Fibronectin, or Poly-L-Lysine) including required volumes and protein concentrations
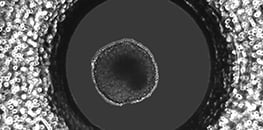
AN 63: Generation and Dynamic Culture of L929 Spheroids in the µ-Slide Spheroid Perfusion (PDF)
Protocol for creating multicellular spheroids in the µ-Slide Spheroid Perfusion with subsequent flow application
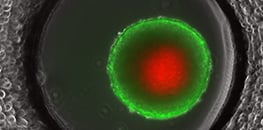
AN 64: FDA/PI Live/Dead Staining Using L929 Spheroids in the µ Slide Spheroid Perfusion (PDF)
Protocol for an FDA/PI fluorescence staining in the µ-Slide Spheroid Perfusion to distinguish living and dead cells in a spheroid