Creating the Gap: Different Approaches
A gap in a cell monolayer can be created using different approaches:
- Insert: Physical cell exclusion is created by placing an insert/stencil on the culture surface before cell seeding
- Scratch: Mechanical cell removal is done by scratching the surface (scratch assay)
For further details and methods on how to create a gap, please have a look at this review:
W.J. Ashby, A. Zijlstra. Established and novel methods of interrogating two-dimensional cell migration. Integr Biol 2012, 10.1039/c2ib20154b
Read article
Creating the Gap Using a Culture-Insert
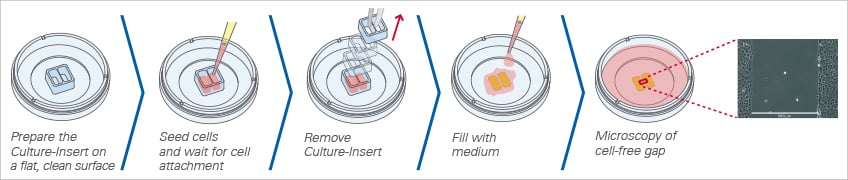
ibidi offers three different Culture-Inserts for gap creation: the ibidi Culture-Insert 2 Well, 3 Well, and 4 Well. Due to the specially designed bottom, the Culture-Inserts stick to the surface, preventing any cell growth beneath the walls. After the removal of the Culture-Insert, the newly created cell-free gap (wound) is clean without any remains. This method allows for the reproducible creation of highly defined gaps without any cells.
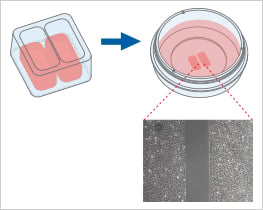
When placed on a flat, clean surface, the Culture-Insert 2 Well provides two reservoirs for culturing cells that are separated by a 500 μm thick wall. The cells are seeded in the reservoirs and cultured until they attach and form a monolayer. Removal of the silicone insert from the surface results in two precisely defined cell patches, which are separated by a zone that is exactly the same width as the separation wall. Cell migration can now be monitored by using live cell imaging or by taking photos at different time points.
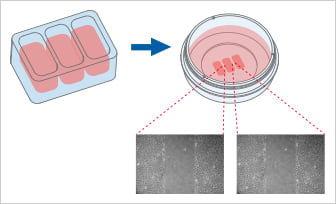
The Culture-Insert 3 Well provides three cell culture reservoirs. It creates two cell-free gaps of 500 µm each and is, therefore, suitable for analyzing the migration of two technical replicates or different cell types.
The Culture-Insert 4 Well provides four cell culture reservoirs. In addition to four 500 µm cell-free gaps, it includes a 1 mm center gap in the middle. The Culture-Insert 4 Well allows for observing the migration of up to four technical replicates or different cell types.
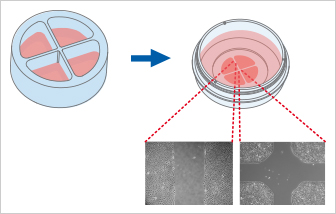
The Culture-Insert 3 Well | 4 Well both enable cell migration analysis after drug treatment or gene silencing/overexpression (e.g., by CRISPR/Cas9, siRNA, mRNA). Importantly, the non-treated control cells can be seeded and analyzed in the same vessel, sharing the same medium.
Unlike other gap creation techniques, the ibidi Culture-Inserts are suitable for 2D invasion assays and co-cultivation assays using two, three, or even four cell types or treatments at the same time.
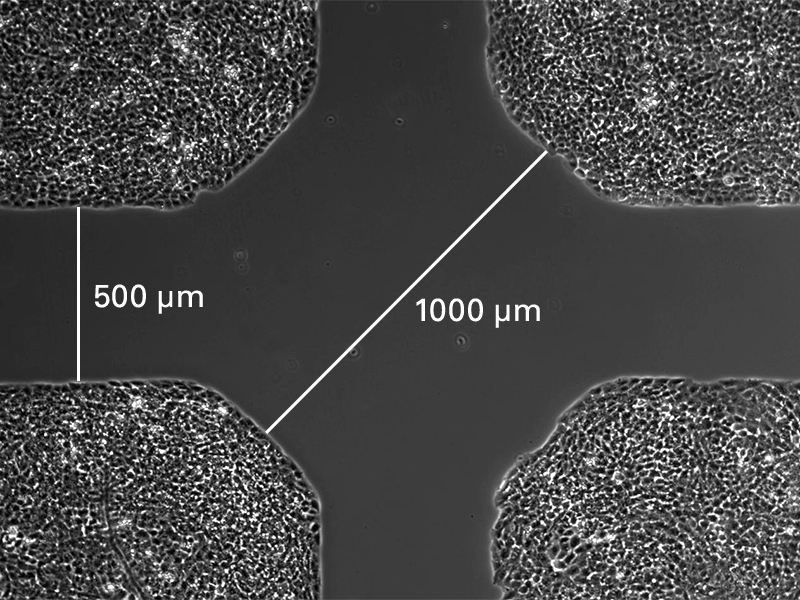
Wound healing and migration assay using the ibidi Culture-Insert 4 Well.
Creating the Gap by Scratching
When doing a scratch assay, the cells are grown until they form a monolayer. Then, the cell surface is manually scratched with a pipet tip or a needle to generate a wound. Wound closure and cell migration are monitored by taking pictures at different time points or live cell imaging.
Regarding reproducibility, this method has certain drawbacks:
- The gap width is highly dependent on the pressure applied to the pipet tip and on its size.
- Scratching removes the surface coating in a non-reproducible way. This alters cell adherence and migration in this area.
- The removed cells form clumps of living and dead cells at the edges of the scratch in a non-reproducible way. The spreading of living cells can overlay the speed of migration.
Culture-Inserts vs. Scratch Assay
At first glance, the methods of scratching and placing a Culture-Insert seem to be two very similar approaches to create a cell-free gap. However, at a closer look, these two methods differ in important aspects that could influence the outcome of the assay:
| ibidi Culture-Inserts | Scratch assay | |
| Gap creation | Cell seeding into designated areas | Scratching the cell surface with a needle or pipet tip |
| Gap size | Defined | Varying (i.e., not reproducible) |
| Gap surface residues | No | Extracellular matrix residues possible |
| Vessel surface damage | No | Yes, due to mechanical scratching on the surface |
| Cell damage | No | Yes, due to scratching the cells |
| Segregation & signaling of necrotic/apoptotic cells | Low | High |
| Internal reference * | Yes | No |

* The internal reference addresses the question of whether or not two opposite cell fronts influence each other. By using the Culture-Inserts, it is possible to measure the speed of:
A: a cell front that is opposite another cell front; and
B: a single cell front that does not have an opposite cell front.
The ibidi Culture-Inserts provide improved reproducibility when compared with a scratch assay
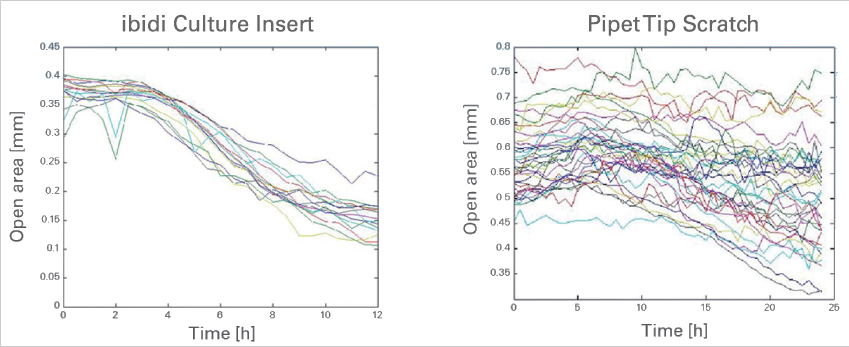
Time-dependent changes of the open area due to cell migration. A comparison between gap creation by using the ibidi Culture-Insert 2 Well (left) and by scratching with a pipet tip (right). Data provided by M. Börries, University Freiburg, Germany.
Read on and learn more about the Principle of Wound Healing and Migration Assays or the Experimental Workflow.




