Recognizing Excellence
in Cell Migration Research
The results are in! We’re thrilled to celebrate the winners of the ibidi Paper Award 2025 for their exceptional research in cell migration. Each awardee will receive 500€ in recognition of their outstanding contributions.
Congratulations to the 2025 Award Winners!
A big thank you to all the researchers who shared their innovative publications and helped make this year’s award a true success. We also extend our deepest appreciation to the esteemed jury—Prof. Dr. Lydia Sorokin, Dr. Nuno Saraiva, and Dr. Guillaume Jacquemet—for their expert evaluation and continued support.

Ilenia Masi for Her Publication in Cell Death & Disease (IF: 8.6)
The β-arrestin1/endothelin axis bolsters ovarian fibroblast-dependent invadosome activity and cancer cell metastatic potential
Ilenia is a postdoctoral researcher at the Institute of Molecular Biology and Pathology of the National Research Council (CNR) in Rome. She earned her MSc in Genetics and Molecular Biology from Sapienza University of Rome in 2018, conducting her thesis at the Istituto Superiore di Sanità. Since 2019, she has worked under Dr. Laura Rosanò, first at the Regina Elena National Cancer Institute, and later at IBPM-CNR. In 2023, she completed her PhD at Sapienza University of Rome, focusing on the interaction between endothelin receptor A and integrin β1 in ovarian cancer progression. Her postdoctoral work focuses on Discoidin Domain Receptors (DDRs) and their role in ovarian carcinoma, with a particular emphasis on tumor-stroma communication. In 2024, she was awarded a one-year AIRC Postdoctoral Fellowship for her project on DDR2 and β-arrestin1. Her research centers on extracellular matrix receptors and their impact on ovarian cancer and its microenvironment.

Ilena Masi, Institute of Molecular Biology and Pathology, CNR, Italy
Why Ilenia’s Publication Convinced the Jury—Nuno Saraiva
This compelling study makes a substantial contribution to our understanding of tumor-stromal interactions, with a particular focus on the activation of ovarian-associated fibroblasts and their invadopodia activity. The authors uncover a novel mechanism involving Endothelin-1 (ET-1) and β-arrestin1 that drives extracellular matrix remodeling, thereby promoting the metastatic potential of serous ovarian cancer (SOC) cells. Notably, they also propose a new therapeutic strategy based on these findings, supported by a proof-of-concept experiment that underscores the potential clinical relevance of their work.
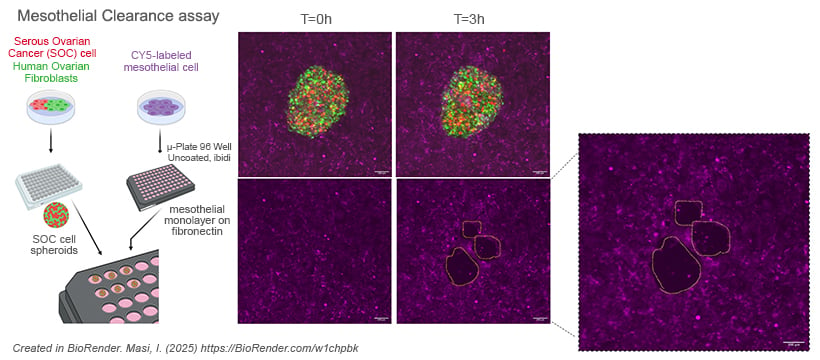
The mesothelial clearance assay was used to assess how cancer cell spheroids invade a mesothelial monolayer, mimicking early metastatic events. Spheroids composed of OVCA433 cells and human ovarian fibroblasts (3:1 ratio) were formed in U-bottom, cell-repellent 96-well plates at 37 °C for 16 hours. Pre-labeled mesothelial cells were seeded on fibronectin-coated ibidi Plates (µ-Plate 96 Well Round, Cat# 89601) and cultured to confluence. Spheroids were transferred onto the mesothelial layer, and live-cell imaging was conducted over 3 hours to monitor cell interactions and mesothelial clearance.
Publication
Del Rio, D., & Masi, I., et al. (2024). The β-arrestin1/endothelin axis bolsters ovarian fibroblast-dependent invadosome activity and cancer cell metastatic potential. Cell Death & Disease, 15, 358.
Syeda M. Bakht for Her Publication in Advanced Science (IF: 15.1)
Human Tendon-on-Chip: Unveiling the Effect of Core Compartment–T Cell Spatiotemporal Crosstalk at the Onset of Tendon Inflammation
Dr. S.M. Bakht earned her Ph.D. in Tissue Engineering, Regenerative Medicine, and Stem Cells from the University of Minho, Portugal, supported by the national research fellowship (PD/BD/135253/2017). Her doctoral work centered on pioneering bioengineering strategies that integrate nanotools, 3D bioprinting, and microfluidic technologies to develop functional and biomimetic tissue models.
Driven by a passion for innovation in biofabrication, Dr. Bakht focuses on merging cutting-edge technologies to create physiologically relevant in vitro systems. Her research combines basic science and clinical applications through interdisciplinary collaboration, aiming to advance the development of next-generation tissue models for both fundamental studies and therapeutic applications.
Currently, Dr. Bakht is a Junior Researcher at CICECO – Aveiro Institute of Materials, University of Aveiro, where she is developing advanced in vitro platforms to investigate spatiotemporal cellular crosstalk in tissue development and disease progression. She remains committed to exploring emerging directions in the ever-evolving field of bioengineering.

Syeda M. Bakht, University of Minho, Portugal
Why Syeda’s Publication Convinced the Jury—Lydia Sorokin
This paper combines a magnetically assisted hydrogel micro structuring in a microfluidic system with tenocytes to recapitulate the unique (unidirectional) 3D architecture of the tendon extracellular matrix (ECM). The use of a multichannel microfluidic system further enables a combination with a vascular component to mimic in vivo inflammatory pathological situations.
This publication, therefore, tackles a difficult in vivo tissue and combines novel material science concepts with microfluidics, which could benefit other fields (the ECM of the cornea or the disordered fibrosis typical of tumors).
Publication
Bakht, S. M., & Pardo, A., et al. (2024). Human Tendon-on-Chip: Unveiling the Effect of Core Compartment–T Cell Spatiotemporal Crosstalk at the Onset of Tendon Inflammation. Advanced Science, 11, 2401170.
Yiyan Lin for Her Publication in Nature Cell Biology (IF: 17.3)
Ras suppression potentiates rear actomyosin contractility-driven cell polarization and migration
Yiyan is a Ph.D. candidate in the laboratory of Dr. Peter N. Devreotes at Johns Hopkins University, where she studies the role of Ras GTPase in regulating cell migration. She earned her bachelor's degree from the Southern University of Science and Technology in China. In her graduate research, Yiyan and her collaborators investigated whether RasGAPs—natural inhibitors of Ras signaling—could suppress cell migration and potentially prevent cancer metastasis. While RasGAP activation successfully inhibited Ras activity, it unexpectedly promoted cell polarization. This surprising outcome suggests that directly targeting Ras may inadvertently enhance cellular behaviors that facilitate metastasis, underscoring the need for careful consideration in the design of anti-Ras therapeutics.

Yiyan Lin, John Hopkins School of Medicine, USA
Why Yiyan’s Publication Convinced the Jury—Guillaume Jacquemet
The study by Yiyan Lin presents a novel perspective on the Ras GTPase, revealing its previously underappreciated role in modulating cell migration. Using optogenetics and high-resolution microscopy, the authors demonstrate that spatial suppression of Ras activity can induce robust cell polarization and enhance motility, challenging assumptions about Ras function. I especially appreciated the careful analysis of the live imaging data, the validation across multiple systems, including Dictyostelium and human neutrophils, as well as computational modeling. The discovery of a link between Ras inhibition and increased migratory capacity has profound implications for basic cell biology. The work is also clinically relevant, indicating that targeting Ras may not always benefit cancer treatment. Altogether, this article makes an outstanding and impactful contribution to cell migration research.
Publication
Lin, Y., & Pal, D., et al. (2024). Ras suppression potentiates rear actomyosin contractility-driven cell polarization and migration. Nature Cell Biology, 26, 1062-1076.
Time-lapse confocal microscopy of vegetative Dictyostelium AX2 cells before and after global membrane recruitment of C2GAPB, a RasGAP. Upon 488 nm blue light stimulation, cytosolic C2GAPB translocated to the membrane, inducing reversible cell polarization and increased migration. Turning off the light restored the less polarized state. Time shown in mins. Scale bar: 10 µm.

Lydia Sorokin
Institute of Physiological Chemistry and Pathobiochemistry, University of Münster, Germany
Lydia Sorokin is Professor of Pathobiochemistry and Director of the Institute of Physiological Chemistry and Pathobiochemisty at the University of Münster, Germany (https://www.medizin.uni-muenster.de/physiolchem/research/ag-sorokin.html). Her interests are the structure and function of extracellular matrix molecules, in particular basement membrane proteins, and their role in different parts of the vascular tree. A particular focus is mechanisms of leukocyte extravasation across postcapillary venules of the brain and how this process differs to that in other tissues, such as the skin or secondary lymphoid organs. Questions addressed include what molecular and physical factors define the sites of leukocyte exits from postcapillary venules, the migration mode, and whether such information impacts on immune cell gene profiles. The group aims to recreate in vitro the molecular and biophysical aspects of the steps that occur in vivo following leukocyte penetration of the endothelial monolayer, when immune cells migrate under constriction and are subjected to differential physical and molecular gradients.

Nuno Saraiva
Lusófona University and Lisbon University, Portugal
Nuno Saraiva holds a PhD in Virology and Cell Biology from Imperial College London, where his research focused on the role of calcium fluxes in cell death and motility. In subsequent research positions, including a postdoctoral fellowship at the University of Cambridge and a Principal Investigator position at CBIOS/Lusófona University, Nuno explored how the dysregulation of calcium signaling and ROS homeostasis modulate cancer cell invasion and metastasis. He is currently the co-head of the Models & Molecular Mechanisms Lab at CBIOS and an Assistant Professor of Cell and Molecular Biology at Lusófona University and the University of Lisbon. Nuno's work has unveiled novel mechanisms through which the Golgi apparatus and mitochondrial metabolism, regulated by proteins such as TMBIMs, impact a cancer cell phenotype. In addition, he developed innovative assays to study the impact of redox modulators on cell migration and collaborated with colleagues to design specific inhibitors targeting cancer cell survival and motility. https://www.cienciavitae.pt//en/0A16-91EA-C570

Guillaume Jacquemet
Turku Bioscience Centre, University of Turku, Finland
Guillaume Jacquemet is an associate professor in bioimaging at Åbo Akademi University in Turku, Finland. He leads the Cell Migration Lab (https://cellmig.org/), which studies cancer cell adhesion and migration using advanced microscopy. Jacquemet has contributed to developing several image analysis tools to study cell migration, including CellTracksColab, ZeroCostDL4Mic, and TrackMate v7, blending biology with technology to enhance research capabilities. His academic journey includes a Ph.D. with Prof. Martin Humphries at the University of Manchester (UK) and a postdoc with Prof. Johanna Ivaska at the University of Turku (FI).
Check out the winners of the ibidi Paper Award 2024 (3D Cell Culture) here.