Förster Resonance Energy Transfer (FRET)
Applications:
FRET determines the precise location and spatial proximity of fluorescently labeled molecules and their interactions in living cells. Using this technique, protein-protein interactions or conformation changes, for example, can be analyzed using a standard widefield or confocal fluorescence microscope. Using specific calcium-sensitive biosensors, FRET can also be applied for the visualization of changes in cellular calcium concentration.
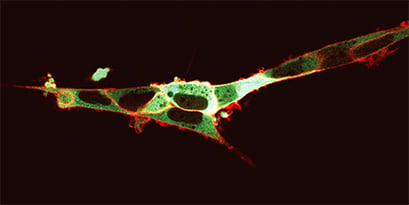
FRET-based visualization of cytoplasmatic calcium concentration. HEK293 cells expressing NK-I on the outer cell membrane and the calcium biosensor Yellow Cameleon 3.6 (YC3.6) in the cytoplasm. Addition of the fluorescently labelled NK-I ligand (SP-TAMRA) results in a red glow of the cell membrane. Upon receptor activation, calcium release induces a change in the YC3.6 fluorescence properties: CFP excitation yields simultaneous CFP and YFP emissions by a FRET phenomenon, appearing as a green glow in the cytoplasm. Provided by M. Roelse, Wageningen, The Netherlands.
Principle:
A donor fluorophore in its excited state can transfer its excitation energy to an acceptor fluorophore in a non-radiative fashion. Typically, this happens through dipole-dipole coupling in a distance of less than 10 nm. Beyond that distance (Förster radius), the two fluorophores show normal fluorescence behavior.
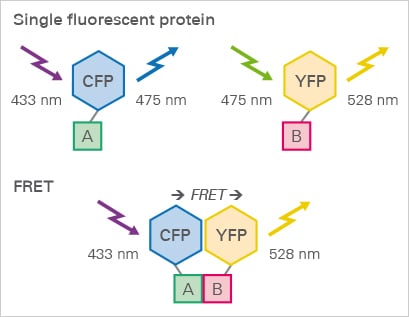
FRET protein interaction assay. A membrane receptor A and its ligand B are tagged with CFP and YFP, respectively. When the ligand binds to the receptor, the YFP is excited by FRET.
H.C. Ishikawa-Ankerhold, R. Ankerhold, G.P.C. Drummen. Advanced Fluorescence Microscopy Techniques—FRAP, FLIP, FLAP, FRET and FLIM. Molecules, 2012, 10.3390/molecules17044047
read abstract
ibidi Solutions:
|  |




