5 Methods to Analyze Chemotaxis:
Recent Studies Explained
ibidi Blog | January 31, 2024 | Abhishek Derle, ibidi GmbH
Chemotaxis, the orchestrated movement of cells in response to chemical signals, is a fundamental phenomenon in biology with far-reaching implications. Researchers across diverse fields leverage this process to explore cellular behavior, immune responses, and developmental mechanisms. In cancer research, chemotaxis sheds light on how cells navigate their environment, contributing to tumor progression and metastasis. In immunology, chemotaxis guides immune cells to infection sites. Beyond basic research, understanding this phenomenon has applications in medicine and biotechnology.
In this blog, we'll explore research articles highlighting diverse approaches to studying chemotaxis. These studies focus on real-time visualization, offering dynamic insights into cellular responses. Real-time measurement captures key events and kinetics, providing a thorough understanding of chemotactic movement compared to endpoint methods. This allows researchers to uncover intricate details shaping our comprehension of this fundamental biological phenomenon.
Learn more about the role of Chemotaxis in Cell Physiology and different Chemotaxis assays.
1. Nanoparticles as "Molecular Knockouts" to Disrupt Chemotaxis [1]
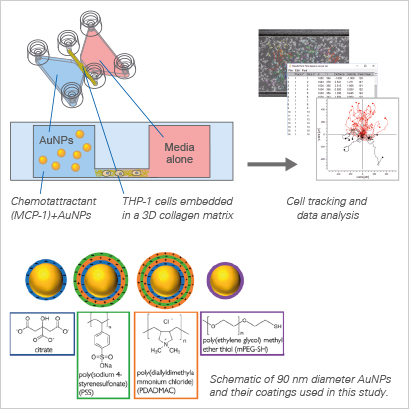
In this interdisciplinary study, Zhang et al. explored the potential of nanoparticles (NPs) to disrupt chemotaxis by acting as "molecular knockouts”. The research aimed to test the hypothesis that NPs could effectively adsorb chemoattractant molecules, thus diminishing their bioavailability and, subsequently, inhibiting chemotaxis. The experimentation utilized a well-established model system involving a human monocytic cell line, THP-1, and its chemoattractant, monocyte chemoattractant protein-1 (MCP-1).
Employing a library of gold nanoparticles (AuNPs) with varying predicted interactions with MCP-1, the study mimicked the in vivo 3D environment of the extracellular matrix in chemotaxis assays. The findings highlighted the profound impact of chemoattractant adsorption on NPs, altering their concentrations near cells and driving changes in cell behavior, all without the need for the NPs to physically enter the cells. This innovative approach provides an understanding of how NPs can act as molecular modulators, opening avenues for novel strategies in the study and manipulation of chemotactic responses.
2. NET-DNA as a Dynamic Chemotactic Factor in Cancer Metastasis [2]
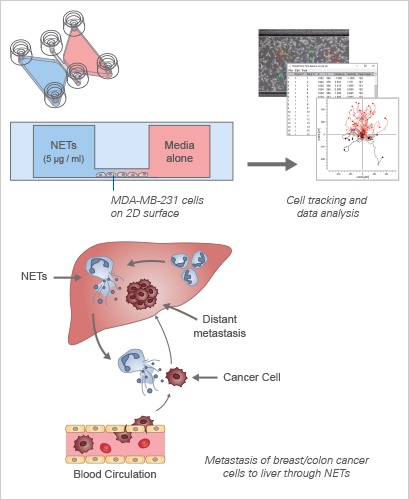
This study by Yang et al. explores Neutrophil Extracellular Traps (NETs) and their role in cancer metastasis. NETs are web-like structures composed of DNA, proteins, and histones, released by neutrophils to trap and neutralize pathogens. The study unveils the abundance of NETs in liver metastases of patients with breast and colon cancers, challenging the conventional notion of NETs as mere microorganism traps. Instead, NET-DNA, a component of NETs, emerges as a dynamic chemotactic factor, actively attracting cancer cells to contribute to the formation of distant metastases.
Through meticulous evaluation using transwell and μ-Slide Chemotaxis assays, the study demonstrates the compelling ability of NET-DNA to promote the migration and adhesion of MDA-MB-231-triple negative breast cancer cells. This effect is further underscored by the dose-dependent enhancement of cell migration, a phenomenon abolished upon treatment with DNase I. The μ-Slide Chemotaxis chamber assay corroborates these findings, revealing the efficient migration of MDA-MB-231 cells towards higher NET-DNA gradients. The findings open new avenues for understanding the intricate interplay between immune responses, NETs, and cancer progression, offering potential targets for therapeutic interventions in metastatic diseases.
3. The Role of Prokineticin-2 in Astrocyte Chemotaxis in CNS Injuries [3]
In this neurobiology study, Neal et al. unravel a novel dimension of Prokineticin-2 (PK2), a chemokine-like signaling protein. Their findings shed light on its crucial role in promoting chemotaxis within astrocytes—an essential mechanism in the context of central nervous system (CNS) injuries. When the CNS is injured or diseased, astrocytes become activated and migrate to the site of damage, contributing to the regenerative response. Building on PK2's known involvement in the activation and migration of systemic cell types, such as macrophages and monocytes, the study explores whether PK2 acts as a chemotactic factor for astrocytes.
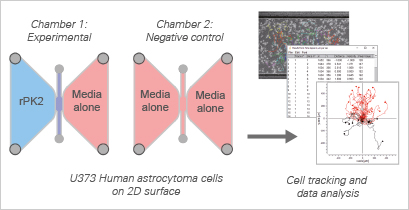
Utilizing the ibidi µ-Slide Chemotaxis and the U373 human astrocytoma cell line, the researchers demonstrate that PK2 indeed serves as a chemotactic factor, significantly enhancing astrocyte migration. In the presence of recombinant PK2 (rPK2), a noticeable increase in the forward migration index (yFMI) was observed, emphasizing a clear directional response towards the reservoir containing PK2. This directional enhancement in migration was accompanied by notable increases in migration velocity and the directionality score, underscoring PK2's impactful role as a stimulator of astrocyte chemotaxis.
Learn more about the μ-Slide Chemotaxis and Experimental Workflow of a Chemotaxis Assay. Explore in more detail Data Interpretation and Data Analysis of Chemotaxis Assays.
4. Platelet Lysate-Loaded PEG Hydrogels Induce Stem Cell Chemotaxis [4]
In this translational study, Chahal et al. investigate the potential of Human Platelet Lysate (PL)-Loaded Poly(ethylene glycol) (PEG) Hydrogels to induce stem cell chemotaxis in vitro. Platelet lysates contain a variety of proteins and growth factors known to mediate cell activity, with some identified as chemoattractants capable of inducing cell migration. The study explores the use of PEG hydrogels incorporating 90 vol% PL to examine their migratory impact on human mesenchymal stem cells (hMSCs).
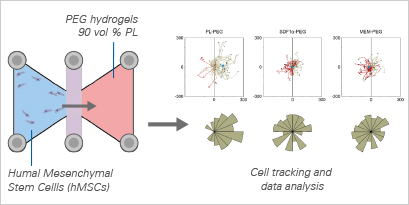
Cell migration studies were conducted using the μ-Slide Chemotaxis. Tracking cell trajectories and migratory parameters revealed that hMSCs exhibited directional migration towards PL-loaded hydrogels, displaying increased velocity and directness compared to controls. This study underscores the bioactive potential of PL-loaded PEG hydrogels, showcasing their capacity to attract stem cells—an exciting prospect for endogenous tissue engineering applications.
5. The Role of RAB35 in Directional Motility and Chemotaxis [5]
In this pivotal study, Corallino et al. unveil the indispensable role of RAB35 in governing optimal directional motility and chemotaxis. Utilizing an RNAi-based approach, the research establishes RAB35 as a key requirement for orchestrating these fundamental cellular processes.
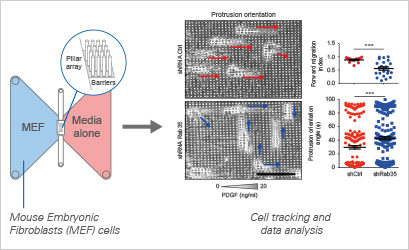
To substantiate this notion, the study delves into the migratory behavior of control and RAB35-silenced mouse embryonic fibroblasts (MEFs) navigating a Platelet-derived growth factor (PDGF) gradient through a meticulously designed array of pillars. This micropillar array, composed of a photocurable hybrid polymer and separated by a 4-μm space, was affixed at the central region of the sticky-Slide Chemotaxis chamber’s self-adhesive underside. Cells were strategically seeded from the inlets of the cell pool area, with the chemoattractant (PDGF) introduced at a concentration of 20 ng/ml in the cell exit area. The dynamic cell motion was recorded using an inverted microscope.
Learn more about the sticky-Slide Chemotaxis designed to allow self-assemblage of the slide setup.
The Chemotaxis and Migration Tool allows for the fast and easy creation of cell trajectory plots, as well as subsequent statistical analysis.
Control cells, exhibiting a functional RAB35 pathway, demonstrated directed chemotaxis toward the PDGF gradient. These cells extended persistent migratory protrusions, predominantly aligned coherently with the gradient direction. In stark contrast, the loss of RAB35 significantly compromised chemotaxis, concurrently reducing the number of cells with protrusions oriented along the gradient. Cells motility was monitored by time-lapse, and manually tracked cells were analyzed by Chemotaxis and Migration Tool. This comprehensive experimental approach reinforces the pivotal role of RAB35 in governing directional cell motility in response to chemotactic cues, providing valuable insights into the molecular intricacies underlying these fundamental cellular processes.
Conclusion
These research articles have provided a captivating exploration of cell behavior in response to chemical cues, spanning diverse fields such as nanotechnology, cancer research, translational studies, neurobiology, and RNAi-based approaches. Collectively, these studies not only contribute to the evolving landscape of chemotaxis research but also inspire novel perspectives and methodologies, paving the way for innovative strategies in medicine.
For more detailed information on how to perform your own chemotaxis assay and analyze the results, visit the ibidi Chemotaxis Application Site.
References
- Zhang, X., P. Falagan-Lotsch, and C.J. Murphy, Nanoparticles Interfere with Chemotaxis: An Example of Nanoparticles as Molecular "Knockouts" at the Cellular Level. ACS Nano, 2021. 15(5): p. 8813-8825.
Read article - Yang, L., et al., DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature, 2020. 583(7814): p. 133-138.
Read article - Neal, M., et al., Prokineticin-2 promotes chemotaxis and alternative A2 reactivity of astrocytes. Glia, 2018. 66(10): p. 2137-2157.
Read article - Chahal, A.S., et al., Human Platelet Lysate-Loaded Poly(ethylene glycol) Hydrogels Induce Stem Cell Chemotaxis In Vitro. Biomacromolecules, 2021. 22(8): p. 3486-3496.
Read article - Corallino, S., et al., A RAB35-p85/PI3K axis controls oscillatory apical protrusions required for efficient chemotactic migration. Nat Commun, 2018. 9(1): p. 1475.
Read article
 (3)
(3)  (1)
(1)



