Organoids: The Miniature Labs
ibidi Blog | May 26, 2025 | Abhishek Derle, ibidi GmbH
Introduction
Organoids may look like simple cell blobs, but they’re rewriting the rules of disease modeling. In labs around the world, tiny 3D clusters of human cells are organizing themselves into structures that resemble miniature organs— revealing the intricacies of tissue development, function, and failure [1]. Welcome to the world of organoids: self-organizing, 3D cell cultures that are reshaping how we study human biology and disease.
Organoids are not just a technical improvement over 2D cultures—they're a paradigm shift. They offer the structural complexity of in vivo models while retaining the accessibility of in vitro experiments. For researchers investigating the mechanisms behind Alzheimer's disease, testing drug responses in colorectal cancer, or modeling the effects of viral infections, organoids provide a unique window into living systems without ever leaving the lab bench.
What Makes Organoids Unique?
One of the most significant contributions of organoids is their ability to serve as more accurate disease models. By closely mimicking the cellular complexity and functionality of human tissues, organoids bridge the long-standing gap between simplified in vitro models and complex in vivo systems. Traditional 2D cultures often fail to capture the three-dimensional organization of tissues, while animal models can fall short in representing human-specific responses. Organoids, however, provide a middle ground—offering the scalability of in vitro systems with physiological relevance closer to that of human organs [2].
What makes organoids truly exciting is their ability to self-organize into structures that reflect not just the architecture but also the developmental dynamics of real tissues. This goes beyond mimicking appearance—they exhibit behaviors and responses that offer a window into how human organs form, adapt, or malfunction. Their relevance is influential in diseases with multifactorial causes and complex tissue interactions.
Organoids can be derived from various cell sources, including patient-specific stem cells, adult tissue stem cells, or even tumor biopsies. This versatility enables both personalized disease modeling and standardized studies of development and pathology.
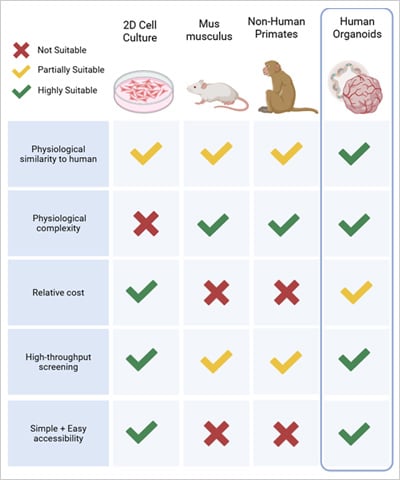
Fig. 1 | Comparison of organoids with other model systems. Adapted from Sun, X., et al.,2023 [3]
Applications of Organoids
Organoids offer greater clinical fidelity than conventional models and are both cost-effective and efficient, enabling more accurate disease simulation and rapid functional testing of drugs. Currently, organoids are primarily utilized in four key areas:
1. Disease Modeling
Organoids have emerged as powerful tools for studying organ development and disease mechanisms in a context that closely mimics human physiology. Derived from patient tissues or pluripotent stem cells, they retain native genetic and functional traits, making them highly suitable for investigating a broad spectrum of conditions—from neurodevelopmental and infectious diseases to metabolic disorders and cancer.
In cancer research, tumor-derived organoids preserve the genetic heterogeneity and architecture of the original tumor, including interactions with stromal and immune cells. This allows researchers to explore mechanisms of tumor progression, invasion, and resistance in a patient-specific manner. Organoids have proven particularly useful in recapitulating complex tumor microenvironments and are now being used to predict treatment responses in real time [4].
In the field of neurological diseases, brain organoids enable studies of human brain development, synaptic formation, and neuroinflammation—processes that are challenging to model in animals [5]. They have provided insight into disorders such as Alzheimer’s disease, autism spectrum disorders, epilepsy, and microcephaly. Notably, brain organoids can reproduce features like tau aggregation and amyloid plaque development, offering a physiologically relevant platform for studying neurodegeneration and testing targeted therapies.
Their ability to model early-stage disease processes, tissue-specific pathologies, and patient variability makes organoids invaluable for uncovering disease mechanisms, identifying novel therapeutic targets, and validating experimental interventions under human-relevant conditions.
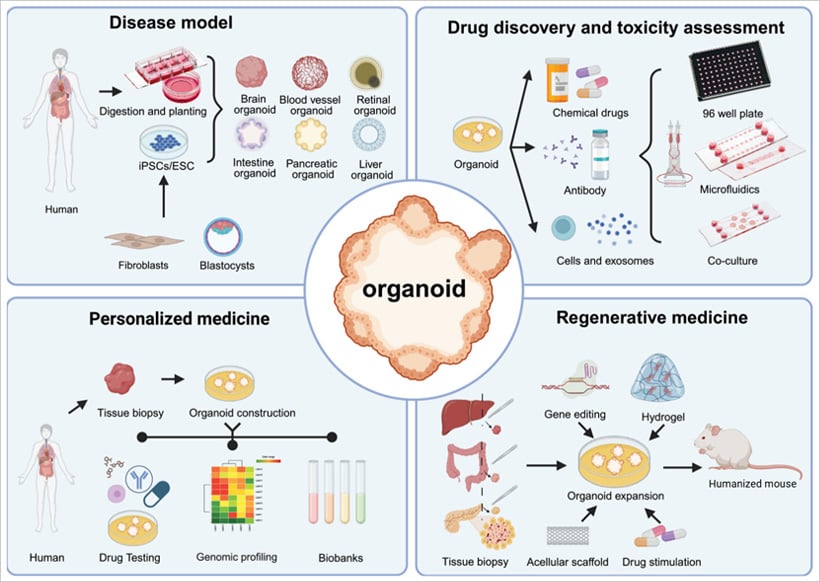
Fig. 2 | Four main applications of organoids. Adapted from Sun, X., et al.,2023 [6]
2. Drug Discovery and Toxicity Screening
The pharmaceutical industry faces enormous pressure to identify safe, effective drugs while minimizing development costs. Organoids provide a solution by offering scalable, high-throughput platforms that mimic real tissue responses [7]. Researchers can now screen hundreds of compounds across patient-derived organoid models to assess therapeutic potential and toxicity. For example, liver and kidney organoids are being employed to detect off-target effects, while tumor organoids are used to predict responses to chemotherapy or targeted agents. Coupling these assays with microfluidics and AI-driven imaging analytics further enhances throughput and precision.
3. Regenerative Medicine
Organoids are not just models—they’re blueprints for tissue replacement. Their use in regenerative medicine includes reconstructing damaged tissues, supporting organ repair, and potentially serving as transplantable building blocks. Advances in bioprinting, scaffold engineering, and co-culture systems with immune or vascular cells have pushed the boundaries of what is possible. While clinical applications remain in the early stages, organoids from intestinal, retinal, and liver tissues have shown promise in preclinical models for restoring lost function and reversing degeneration [8].
4. Personalized Medicine
One of the most transformative applications of organoids is tailoring treatments to individual patients. By cultivating organoids from patient-derived tissues, clinicians can test drug efficacy and resistance in vitro before administering therapies. This approach is already showing clinical value in cancers such as colorectal, breast, and pancreatic, where tumor heterogeneity often leads to variable treatment outcomes. In cystic fibrosis, for instance, organoid-based assays have guided therapy decisions when genetics alone fell short. To illustrate the impact of this approach, let’s look at a real-world example:
Case Study: Cystic Fibrosis and Personalized Drug Testing [9]
A compelling real-world example of organoid application is found in cystic fibrosis (CF) research. Scientists have developed intestinal organoids from patient-derived stem cells and use a functional swelling assay to evaluate the effectiveness of CFTR-modulating drugs. These organoids respond to effective treatment by swelling due to ion and water influx—a direct visual cue that a drug is working.
This assay has enabled clinicians to predict drug responses for individuals with rare or poorly understood CFTR mutations. In many cases, it has guided personalized therapy where genetic testing alone offered no clear path forward. This model illustrates the power of organoids in drug testing and showcases their clinical utility in precision medicine.
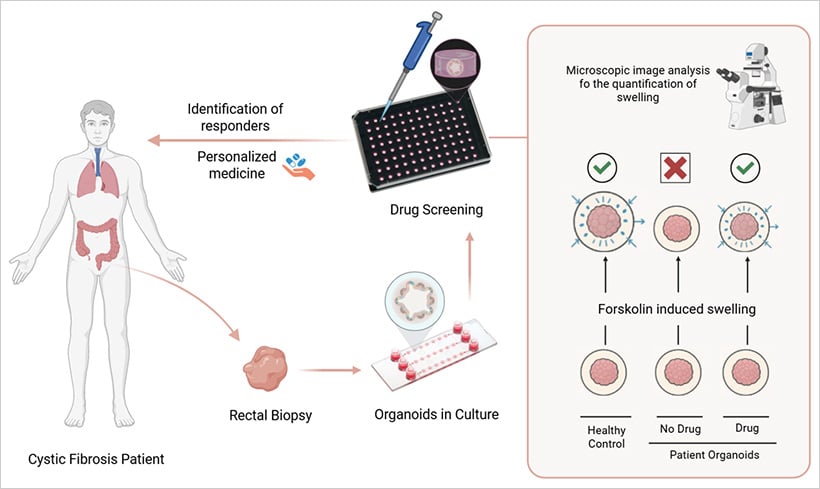
Fig. 3 | Evaluation of CFTR function in patient-derived organoids
Enabling Technologies: The Role of Smart Platforms
Organoid research depends as much on supportive technologies as on cells themselves. Modern platforms enable precise gel handling, long-term perfusion, and advanced imaging—all critical for reproducible and physiologically relevant experiments.
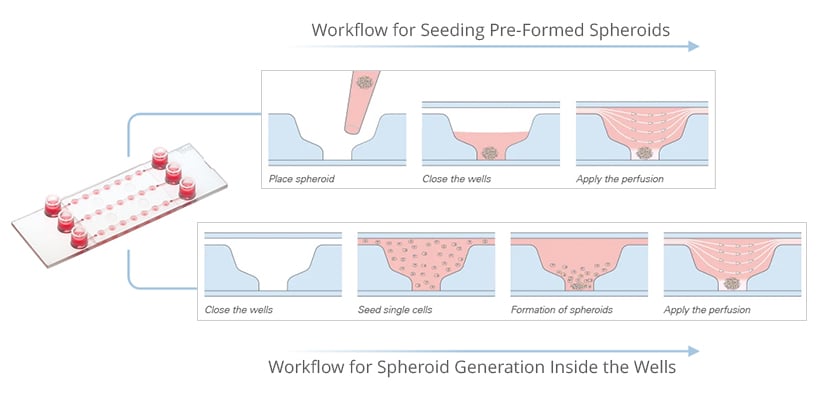
Microfluidic tools like the µ-Slide Spheroid Perfusion offer controlled flow to mimic in vivo conditions, supporting real-time studies of growth, drug responses, and tissue organization.
ibidi’s µ-Patterning technology allows spatially defined cell adhesion using non-adhesive surfaces with patterned spots. This setup is ideal for generating uniform spheroids, studying cell behavior, or performing CAR-T cell-killing assays with high-resolution imaging.
Lastly, the µ-Plate 96 Well 3D combines high-throughput capacity with minimal gel use and superb optical quality—making it ideal for screening, 3D cell culture, and live imaging in a standardized, efficient format.
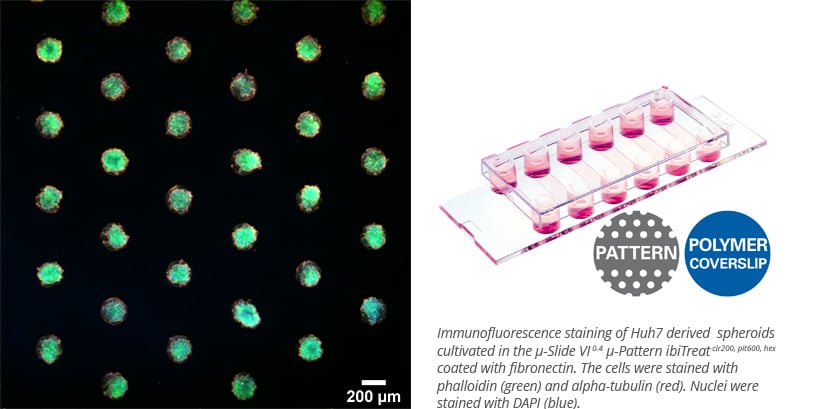
Beyond Single Organoids: Assembloid Systems
While traditional organoids offer insights into individual organ behavior, many human diseases involve crosstalk between multiple tissues. This has led to the emergence of multi-organoid systems, also known as assembloids, which combine different organoids—such as brain–spinal cord, or gut–liver—to better replicate physiological interactions. These models are increasingly used to study development, systemic drug effects, hormone signaling, and immune-organ communication on a single platform. Their complexity opens new possibilities for modeling diseases that span multiple organ systems and building more holistic, body-on-a-chip platforms.
One recent example involves a cardiorenal unit where cardiac and kidney organoids derived from hiPSCs were combined on a microfluidic chip [10]. Using the µ-Slide III 3D Perfusion, researchers maintained the structural and functional integrity of both organoids under static and flow conditions. The system demonstrated how organoid co-culture under perfusion can enable future studies of inter-organ signaling and disease mechanisms—moving the field closer to fully integrated organ-level modeling.
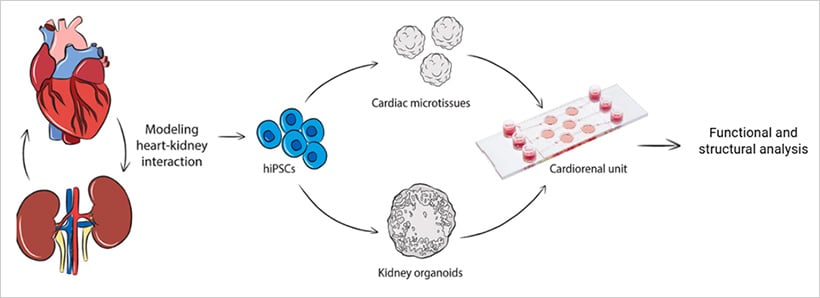
Fig. 4 | Dual organoid system for studying inter-organ crosstalk[10]
Conclusion
As science pushes the boundaries of what we can replicate outside the human body, organoids stand at the frontier of innovation. These tiny, organ-like cell clusters are helping scientists do everything from modeling diseases to testing new drugs, all without leaving the lab.
And as we keep improving these models and start mixing in AI, omics, and bioengineering, we're heading toward a future where personalized, organ-level insight shapes medicine from bench to bedside.
To learn more about spheroids, organoids, and 3D cell culture, visit our 3D Cell Culture Application Site.
References
- Yi, S.A., et al., Bioengineering Approaches for the Advanced Organoid Research. Adv Mater, 2021. 33(45): p. e2007949.
- Kim, J., B.-K. Koo, and J.A. Knoblich, Human organoids: model systems for human biology and medicine. Nature Reviews Molecular Cell Biology, 2020. 21(10): p. 571-584.
- Sun, X., et al., A narrative review of organoids for investigating organ aging: opportunities and challenges. Journal of Bio-X Research, 2023. 06(01): p. 3-14.
- Fang, Z., et al., The role of organoids in cancer research. Experimental Hematology & Oncology, 2023. 12(1): p. 69.
- Birtele, M., M. Lancaster, and G. Quadrato, Modelling human brain development and disease with organoids. Nature Reviews Molecular Cell Biology, 2025. 26(5): p. 389-412.
- Yao, Q., et al., Organoids: development and applications in disease models, drug discovery, precision medicine, and regenerative medicine. MedComm, 2024. 5(10): p. e735.
- Vandana, J.J., et al., Human pluripotent-stem-cell-derived organoids for drug discovery and evaluation. Cell Stem Cell, 2023. 30(5): p. 571-591.
- Wu, Y., et al., Application of Organoids in Regenerative Medicine. Stem Cells, 2023. 41(12): p. 1101-1112.
- Dekkers, J.F., et al., Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Science Translational Medicine, 2016. 8(344): p. 344ra84-344ra84.
- Gabbin, B., et al., Heart and kidney organoids maintain organ-specific function in a microfluidic system. Mater Today Bio, 2023. 23: p. 100818.
 (3)
(3)  (0)
(0)



