High-Content Screening: From Microscopy to Medicine
ibidi Blog | May 28, 2024 | Abhishek Derle, ibidi GmbH
In the field of drug discovery, you may have come across the terms High-Content Screening (HCS) along with its close companions: High-Content Imaging (HCI) and High-Content Analysis (HCA). But what exactly does "High-Content" mean? And how are these concepts different from each other?
These terms are often mentioned alongside the foundational concept of High-Throughput Screening (HTS), which employs a vast network of automated robotic systems capable of testing anywhere from hundreds of thousands to millions of compounds, efficiently narrowing down potential drug candidates. Building upon the foundation of HTS, High-Content Screening (HCS) takes these capabilities further by incorporating advanced microscopy and imaging techniques where, instead of just one endpoint, you measure many different endpoints or outputs.
This blog explores how High-Content Screening, an umbrella term encompassing High- Content Imaging and High-Content Analysis, enhances the drug discovery process by capturing phenotypic data showing cellular response(s) to compounds.
High-Content Imaging (HCI): Enhancing Visualization
At the heart of High-Content Screening is High-Content Imaging (HCI), an image-based high-throughput method that utilizes microscopy techniques like confocal microscopy, live cell imaging, and automated multicolor fluorescence imaging. This approach enables the detailed capture of cellular samples to simultaneously analyze multiple molecular features in individual cells, in both 2D and 3D cell cultures. In essence, HCI enhances the depth and accuracy of cellular analysis in drug discovery, enabling scientists to gain valuable insights from cellular responses to potential therapeutic compounds. Below are the key applications of HCI in High-Content Screening:
1. Assay Development
By providing detailed visualization, HCI helps researchers to design robust assays that can accurately measure specific biological processes or endpoints, such as disease-relevant cell-based assays. These assays provide precise measurements and monitor phenotypic changes, facilitating the optimization of early discovery efforts. For instance, the high-Throughput tube formation assay serves as an in-vitro tool for accessing angiogenesis in an easy, cost-effective, and reproducible manner, allowing the tube formation ability of cells to analyze the pro- or anti-angiogenic potential of various drugs. Similarly, Wound Healing and Migration Assays facilitate the analysis of cell migration under different conditions, making them valuable additions to the researcher's toolkit.
Apart from this, HCI empowers researchers to generate preclinical data that is both predictive and translatable to in-vivo effects, especially when integrated with patient-relevant 3D organoid cultures. This synergy between advanced imaging technology and 3D organoid models enhances the reliability of preclinical assessments.
With the µ-Plate 96 Well 3D, ibidi offers a solution for high throughput tube formation and 3D Cell Culture assays. It offers brilliant cell visualization without gel meniscus formation which ensures reproducible cell culture conditions. The ready-to-use Culture-Insert 2 Well 24 is ideal for reproducible high throughput wound healing and migration assays. It consists of silicone Culture-Inserts with a defined cell-free gap that are already inserted into the µ-Plate 24 Well.
2. Large-Scale Transfection Assays
HCI is widely employed in large-scale transfection assays, serving as an important tool for evaluating the efficacy and effects of gene delivery into cellular systems. Through its ability to visualize and quantify gene expression, HCI enables researchers to fine-tune transfection protocols, understand gene function, and explore molecular interactions.
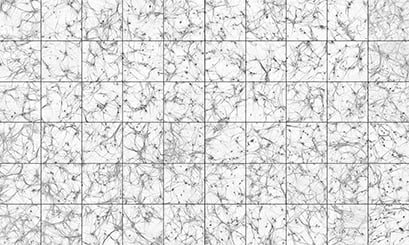
Human induced pluripotent stem cell (hiPSC) derived neurons stained with MAP2, a marker of the cell body and proximal dendrites. The neurons were differentiated via neurogenin 2 induction, and co-cultured with mouse glia for 30 days. Each square was recorded from a separate well of an ibidi µ-Plate 96 Well Square. The images were recorded on a High-Content confocal imaging system with a 10x objective. Image by Chris Hempel, Q-State Biosciences, Inc., Cambridge, MA, USA.
3. Compound Screening
Through HCI, researchers can scrutinize the impact of compounds on diverse aspects of cellular behavior, encompassing cell morphology, protein expression, and subcellular localization, among others, all evaluated simultaneously. This multifaceted analysis assists in the pinpointing compounds possessing desired properties, advancing drug discovery efforts with efficiency and precision.
For example, the study conducted by Schuth et al. highlights the potential of personalized PDAC co-culture models for comprehensive drug response profiling and elucidating the molecular mechanisms contributing to tumor stroma-mediated chemoresistance. Utilizing High-Content Imaging techniques was instrumental in unraveling these intricate interactions within the co-culture environment, offering valuable insights into PDAC pathobiology and therapeutic strategies.
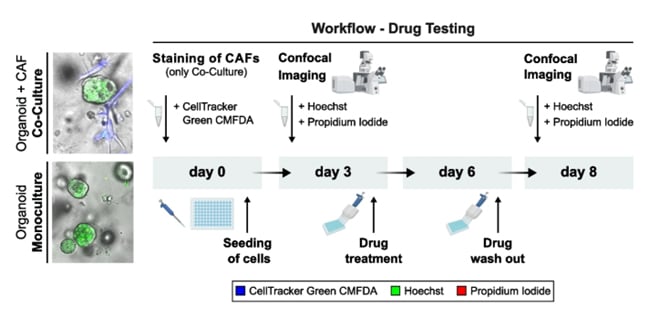
Schematic overview of the established drug test workflow for PDAC-PDO mono- and PDAC-PDO/CAF co-cultures.
4. Toxicology Studies & Safety Assessment
HCI emerges as a cornerstone in toxicity studies, allowing for the assessment of the safety profiles of potential drug compounds or environmental factors. It facilitates monitoring cellular viability, apoptosis, oxidative stress, and other toxicological endpoints, enabling the identification of compounds or conditions that may pose risks to cells or tissues.
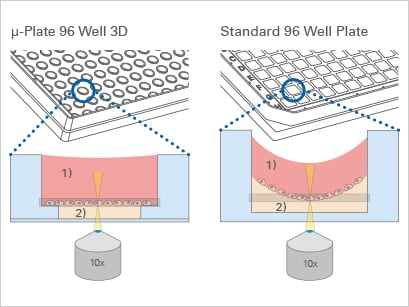
It is crucial that for High-Content Imaging (HCI), the bottom of the plate must be both thin and flat to facilitate light transmission necessary for image creation. Before using a plate for HCI, researchers need to verify that it meets imaging-quality standards.
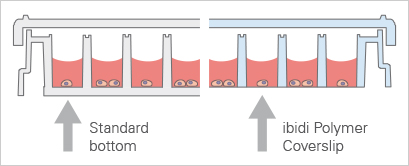
Standard 96 Well Plate With a 1 mm thick bottom made of polystyrene, not suited for high-resolution or fluorescence microscopy. | ibidi µ-Plate 96 Well With a flat ibidi Polymer Coverslip #1.5 bottom (180 µm, +10/–5 µm), ideal for for high-resolution or fluorescence microscopy. |
ibidi provides specialized microtiter plates designed to meet the rigorous demands of these applications. Our plates feature black square or round wells with a flat, clear bottom, ensuring optimal imaging quality. They are available with a #1.5 ibidi Polymer Coverslip (µ-Plate 96 Well Square and µ-Plate 96 Well Round) or a #1.5H Glass Coverslip bottom (µ-Plate 96 Well Square Glass Bottom , µ-Plate 96 Well Round Glass Bottom, and µ-Plate 384 Well Glass Bottom)
5. Live Cell Analysis
HCI is well-suited for live cell analysis, enabling real-time monitoring of dynamic cellular processes. It facilitates the study of cellular behaviors, such as cell migration, cell-cell interactions, and intracellular signaling events. HCI allows researchers to capture time-lapse images and track changes in cellular phenotypes over extended periods, providing valuable insights into cellular dynamics and responses to stimuli.
ibidi offers Stage Top Incubation Systems for high-throughput live cell imaging using microplates that have an ANSI/SLAS (SBS) standard format. This system enables effortless easy live cell imaging on every inverted microscope, providing precise control of temperature, humidity, and CO2 and O2.

High-Content Analysis (HCA): Decoding Complex Data
High-Content Analysis refers to using automated image analysis software to analyze the vast amounts of data generated by High-Content Screening. HCA can analyze multiple parameters from each cell in an image, processing thousands of cells per experiment. This includes quantifying changes in cell shape, volume, texture, and fluorescence intensity across a range of wavelengths. HCA has evolved to encompass multicellular configurations like 3D spheroids and co-cultures, as well as the ability to multiplex, enabling the simultaneous monitoring of various features within a single microenvironment in each well.

Workflow for High Content Screening
Recent Trends in High-Content Screening
One of the key trends in recent HCS developments is the integration of machine learning and artificial intelligence (AI). These technologies enhance the analysis capabilities of HCS systems, enabling them to recognize patterns and features in cellular images that might be too subtle or complex for traditional analysis methods. Machine learning algorithms, for instance, are being used to improve the accuracy and speed of image analysis, including the ability to automatically classify cell types, quantify phenotypic changes, and predict cellular responses to different treatments.
Another significant trend is the advancement in imaging technologies themselves. There has been a push towards higher resolution and faster imaging systems that can capture detailed cellular dynamics in real-time. Super-resolution microscopy techniques are now more frequently paired with HCS platforms, providing unprecedented insights into the cells’ molecular machinery. Additionally, multispectral imaging techniques allow for the simultaneous detection of multiple fluorescent markers, facilitating complex studies on cellular interactions and functions.
The application of HCS is also expanding in the scope of biological complexity it can handle. Recent innovations have led to the development of systems capable of analyzing multicellular structures, such as organoids and 3D cell cultures. These 3D cultures, which better mimic the natural environment of cells within the human body, provide new ways to screen drugs and understand disease mechanisms with higher relevance to clinical outcomes.
References
Schuth, S., Le Blanc, S., Krieger, T.G. et al. Patient-specific modeling of stroma-mediated chemoresistance of pancreatic cancer using a three-dimensional organoid-fibroblast co-culture system. J Exp Clin Cancer Res 41, 312 (2022).
Read article
 (1)
(1)  (0)
(0)



