The Placenta: Here for a Good Time, Not a Long Time
Molecular Insights Into Placenta Development and Pathology
ibidi Blog | August 24, 2022 | Derek Sung, University of Pennsylvania
Perhaps no organ is as underappreciated as the placenta. It is the first organ that forms, but in the grand scheme of life, it stays with us only for a short period of time. It is a fascinating organ consisting of both maternal and fetal parts that interact in complex ways to ensure efficient nutrient exchange to nourish the fetus. For such a short-lived organ, it has profound, lifelong consequences on our health. Defects in the placenta have been linked to congenital heart defects, neurodevelopmental disorders, and even metabolic diseases. Considering all that it does in promoting fetal growth, it seems almost comical that it's often unceremoniously tossed in the biohazard bin after birth.
Why I Became Interested in Placental Biology
I first remember getting interested in placental biology during my clinical rotation, as a medical student, on the labor and delivery floor. Watching a live birth is one of those unforgettable experiences we, as medical students, are privileged to experience as a part of our training. The majority of pregnancies and births are uncomplicated and go smoothly, but as doctors-in-training, we learn about the good and the bad. One of the most common pregnancy complications I saw was preeclampsia. Preeclampsia is a hypertensive pregnancy disorder estimated to affect 5-7% of pregnancies globally, contributing to 70,000 maternal deaths and 500,000 fetal deaths every year1. This means that preeclampsia affects up to one in fourteen pregnancies. Preeclampsia can cause maternal organ damage, fetal growth restriction, and preterm birth, among other complications. Despite its significant impact on maternal and fetal health, there is currently no cure for preeclampsia except delivering the baby and placenta.
The Placenta is a Mysterious Organ
When I asked other doctors around me what causes preeclampsia, there seemed to be no consensus other than the fact that preeclampsia is related to placental dysfunction. This was surprising and unsatisfying to me, considering preeclampsia is so common. It wouldn't be out of the ordinary for me to see two or three preeclamptic patients on the labor and delivery floor in a single shift. How could a disorder that affects so many women with potentially devastating complications have no treatments and be so poorly understood? This was my motivation for pursuing work on how the placenta develops as a part of my thesis work.
Understanding Placenta Pathology and Development on a Molecular Level
Insight into placenta pathology requires an understanding of how the placenta forms and functions. Early during placental development, specialized fetal cells called trophoblasts must connect to and 'replace' maternal blood vessels in order to funnel maternal blood into the placenta for nutrient exchange. How exactly trophoblasts manage to accomplish this is a big mystery. Weknow that trophoblasts carry out many of the same functions as endothelial cells in that they form vessels and carry blood. Trophoblasts even express many conventional molecules of endothelial cells, such as vascular endothelial (VE-)cadherin, an endothelial cell-cell adhesion protein. This adaptation of endothelial traits has dubbed trophoblasts as undergoing "vascular mimicry." Could these endothelial genes be helping trophoblasts connect to maternal blood vessels? What happens if they lose this ability? This was the question we asked in our recent paper published in eLife 2.
How VE-cadherin Contributes to Trophoblast Function
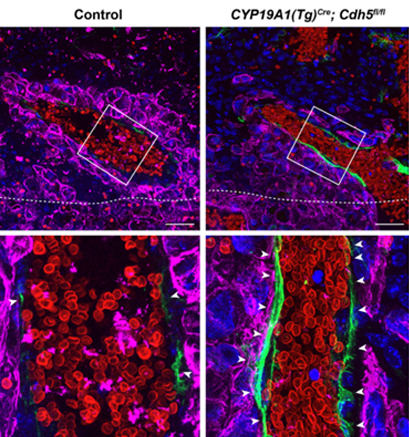
We tested the role of VE-cadherin by creating a mouse model in which we deleted VE-cadherin from trophoblasts. This resulted in a few things. First, embryos were growth restricted, and the placentas were smaller. Second, when we looked at these placentas by histological staining, we found significantly less maternal blood in the placenta. These results suggested there may be an issue with how trophoblasts are connecting to maternal blood vessels. Finally, immunofluorescence staining showed significant defects in trophoblasts' ability to invade the placenta's maternal side and connect to maternal blood vessels. In fact, we saw that trophoblasts lacking VE-cadherin were completely unable to displace maternal blood vessels, restricting the ability of maternal blood to be effectively shunted into the placenta ( Figure 1 ). This explained why the embryos were much smaller than expected – they weren't getting the nutrients they needed.
|
Figure 1. Immunofluorescence staining of normal (left) and trophoblast VE-cadherin knockout (right) mouse placentas for trophoblasts (Cytokeratin 8, magenta), endothelial cells (Endomucin, green), and red blood cells (TER119, red). Nuclei are stained with DAPI (blue). White arrowheads point to persistence endothelial cells in VE-cadherin knockout placentas that should not be there. Images in bottom row are correlated to white boxes in top row. Image used with permission from Sung et al. 2022, eLife |
A New Mouse Model of Preeclampsia
To ensure that what we were seeing was physiologically reflective of what's going on in preeclampsia, we used an ultrasound probe to assess blood flow through the umbilical cord. Similar to what we do in patients, we can calculate the umbilical cord resistance and pulsation using various ultrasound parameters. Our mice exhibited increased umbilical resistance and pulsation, similar to the clinical parameters shown by patients with preeclampsia and demonstrating that something was wrong with blood flow in the placenta. We were even able to detect slowing heart rates in our embryos, indicating that our embryos were in distress. These findings are consistent with a preeclamptic-like phenomenon and suggest that VE-cadherin, and vascular mimicry in general, may play an important role in trophoblasts and preeclampsia pathogenesis.
Future Directions
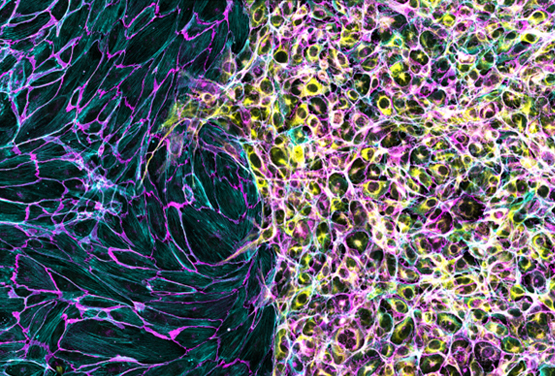
So why is VE-cadherin specifically important for trophoblasts? One potential reason is that VE-cadherin might actually mediate how trophoblasts interact with endothelial cells in maternal blood vessels. Both cell types express VE-cadherin, so VE-cadherin expression in trophoblasts may actually help them adhere to – and eventually displace – endothelial cells in maternal blood vessels. Currently, we are developing in vitro systems to model this interaction. One of the ways we're doing this is with ibidi Culture-Inserts . I've been culturing endothelial cells on one side and trophoblasts on the other and letting them migrate toward each other ( Figure 2 ). I've written about my absolute obsession with this product before (read about it here ), but the ability to culture two different cell types and watch how they interact just highlights another unique advantage. These types of experiments have been absolutely instrumental in determining the mechanism underlying our findings.
|
Figure 2. Endothelial cells (left) and trophoblast cells (right) were cultured in an ibidi µ-Dish 35 mm, high with a Culture-Insert 2 Well. Cells were allowed to migrate towards each other after removal of the insert prior to fixation, staining, and imaging. Cells were stained with an antibody against VE-cadherin (magenta), phalloidin for F-actin (cyan), and Cytokeratin 8 (yellow). Image used with permission from Derek Sung. |
We have only begun to scratch the surface of placental biology. My hope is that the next generation of scientists will take a particular interest in women's and reproductive health, as it is an area of science and medicine that is severely understudied. My dream is to one day open my own lab to study placental development that may lead to new therapies for patients with placental-related disorders.
References:
- Rana S, Lemoine E, Granger J, Karumanchi SA. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ Res . 2019;124(7):1094-1112. doi:10.1161/CIRCRESAHA.118.313276
- Sung DC, Chen X, Chen M, et al. VE-cadherin enables trophoblast endovascular invasion and spiral artery remodeling during placental development. Elife . 2022:1-17.
Derek Sung — MD/PhD Student Derek is an MD/PhD student at the University of Pennsylvania School of Medicine, working in the fields of developmental and cell biology. His research investigates mechanisms of vascular development and he has an expertise in immunofluorescence staining and confocal microscopy. Check out his Instagram Channel @immunofluorescence . |
|
 (5)
(5)  (0)
(0)