5 Tips for Preparing Primary Cells from the Peripheral Nervous System for Live Cell Imaging
ibidi Blog | August 13, 2021 | Flavia Millesi, Medical University Vienna
Live cell imaging accounts for one of the most fascinating techniques in modern cell culture. It not only allows us to see live snapshots of our cells like other imaging methods but is ultimately the only way for us to see our cells in action. To understand how they move and migrate, how they communicate, how they undergo cell division, and how they excrete, secrete, phagocytize and feed. Live cell imaging helps us to understand biological processes. But as with all methods, there are some things to look out for when performing live cell imaging, starting from cell culture parameters over technical aspects to the best way to analyze the data.
Our team at the laboratory of Professor Christine Radtke, Medical University of Vienna, has been using live cell imaging since right from the beginning of our year-long research in Vienna. We employ it to investigate the interactions between peripheral nervous system cells such as nerve cells, Schwann cells, neuronal fibroblasts, and various biomaterials used for tissue reconstruction. As time is a critical factor in nerve regeneration, we are particularly interested in enhancing the migratory potential of Schwann cells, which need to quickly reach and bridge the injury site to guide the regrowing axons.
I highly recommend that everybody, who is interested in cell behavior and processes, uses live cell imaging for their research. Here are some tips from us to facilitate your start into getting to know your cells on a whole new level.
1. Make Sure You Have the Right Cell Culture Chambers
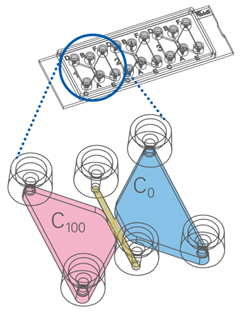
First things first, you will need to decide what elements are visually meaningful. Do you simply want to visualize your cells migrating? Or do you want to investigate whether your cells change their migratory direction to a specific growth factor for example? There are a wide variety of cell culture chambers available for live cell imaging and especially ibidi offers various options perfectly suited for your needs. For example, the µ-Slide Chemotaxis chambers (Figure 1) allow real-time chemotaxis measurements by providing a cell chamber that allows various factors to be added. The cells are seeded in the middle between the chambers and it can be analyzed to which side the cells preferably migrate. For the most part, we use the µ-Slide 4 Well for our live cell imaging experiments as their larger areas allow observation of multiple positions in one well. Another option is the µ-Slide 4 Well Ph+ , which has a special intermediate plate for excellent phase contrast and high-end fluorescence microscopy. There are many various chamber models available, many of which we haven't tried yet, but definitely will in the future. Let me know if you have experience with any other chamber slides and how to use them! |
Figure 1: ibidi µ-Slide Chemotaxis |
2. All About the Cells: Carefully Plan Your Sample Preparation
Next, it's important to contemplate how to prepare the cells. Depending on which cell type, how fast they move, and how quickly they grow in numbers, various parameters need to be determined. Schwann cells for example need some time to settle, so it makes sense to start the live cell imaging experiments a few hours after seeding. Moreover, some cells tend to be influenced by cell-cell contact, so it's important to think about the right cell seeding number differing on what one is investigating. Finally, it is also crucial to decide if an additional coating is required for the cells.
3. Keep Your Cells Alive and Happy on the Microscope
As soon as the cells are ready, we can go to the microscope. Unfortunately, not all microscopes can be used for live cell imaging. It is important that they are equipped with a built-in incubator regulating the amount of CO 2 and temperature in order to keep the cells alive. We are using an Olympus IX83 combined with the ibidi Stage Top Incubation System (see Figure 2). It offers us precise control of temperature and CO 2 as well as humidity. We usually perform our live cell imaging experiments under a constant temperature of 37°C and 5% CO 2 with 50% humidity. There are two different heated plates available, one for a single chamber slide and another one with which you can image four chamber slides at once. Perfect for numerous experiments.
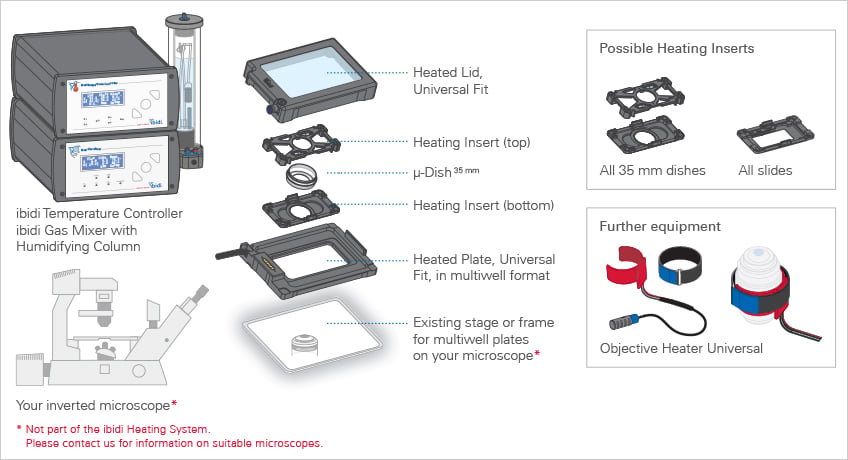
Figure 2: ibidi Stage Incubation System
4. Choose Appropriate Microscopy and Recording Settings
Once the chamber slides are secured in the plate under the heated lid, we can start the experiment. Important things to consider are the magnification of the microscope, how often the system should take pictures, and for how long. For example, as Schwann cells are fast-moving cells, it makes sense to use a lower magnification than fibroblasts which move much slower and migrate for shorter distances. We are using the software cellSens Olympus Corporation to take a picture every ten minutes for at least 10 hours which are good settings for migration experiments related to speed and covered distance of the cells. If you are interested in how the cells move or even in something completely different, like how cells take up particles, it might make sense to shorten the time between pictures taken.
5. Quantify Your Experiment by Analyzing the Data
After the experiment ended, the software, in our case, presents us with .vsi files, which we usually covert into .avi videos and .tiff image sequences. Then, using ImageJ, we track selected cells using the Manual Tracking plugin by following and clicking on single cells in every frame. This results in an individual migration path for every cell. Afterwards, evaluate the results with the ibidi Chemotaxis and Migration Tool , which allows the calculation of an average velocity, average total and Euclidean (effective) distance, as well as the directionality index for each cell (see Figure 3).
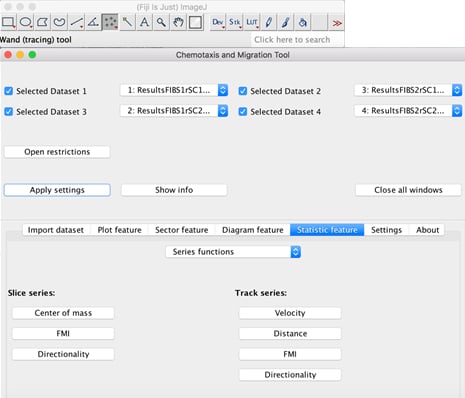
Figure 3: Interface of the ibidi Chemotaxis and Migration Tool plugin.
In the end, this is the kind of amazing data you can get out of it:
Video showing migrating Schwann cells
References:
F. Millesi, T. Weiss, A. Mann, M. Haertinger, L. Semmler, P. Supper, D. Pils, A. Naghilou, C. Radtke. Defining the regenerative effects of native spider silk fibers on primary Schwann cells, sensory neurons, and nerve-associated fibroblasts. FASEB Journal. 2021, 10.1096/fj.202001447R
Flavia Millesi, MSc, BSc -- PhD Student
|
|
 (0)
(0)  (0)
(0)