Shear Genius:
Microfluidics in Atherosclerosis Research
ibidi Blog | November 20, 2024 | Abhishek Derle, ibidi GmbH
Introduction
Atherosclerosis is a chronic disease that affects millions worldwide, contributing to heart attacks, strokes, and other cardiovascular complications. At the core of this disease is the gradual buildup of plaques inside arterial walls, leading to restricted blood flow, inflammation and, eventually, critical events like heart attacks. A key factor in the development of atherosclerosis is shear stress, the mechanical force exerted by blood flow on the arterial walls. In regions with laminar blood flow, endothelial cells tend to function normally. In contrast, endothelial dysfunction occurs in areas of disturbed or oscillatory flow—often found in arterial bifurcations—making these regions prone to plaque formation. [1]
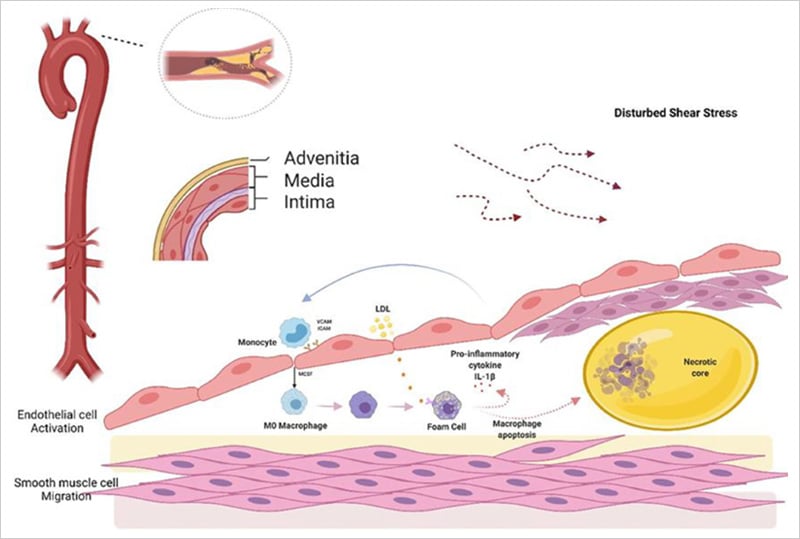
Pathogenesis of Atherosclerosis
Shear stress initiates atherosclerosis by activating endothelial cells in the intima layer. Normally protective, these cells become permeable under disturbed shear stress, allowing monocytes and LDL entry. Oxidized LDL forms foam cells, releasing pro-inflammatory factors that, through ICAM and VCAM, recruit more monocytes, creating a feedback loop. This ultimately results in a necrotic core capped by smooth muscle cells, forming a fibrous cap. The figure was reproduced under CC BY 4.0 from Hua Y et al., Front. Cardiovasc. Med. 2022;9:831847.
Traditional research models, such as animal studies and static cell cultures, face limitations in replicating the complex dynamics of human vascular physiology. Animal models often fail to mimic human conditions accurately, and static cultures lack essential mechanical forces like shear stress. These limitations hinder the ability to fully understand how endothelial cells behave in real physiological settings, creating a need for more advanced in vitro systems. Microfluidic perfusion systems address these challenges by recreating the physiological conditions required to simulate disease progression. In this blog, we will explore how these systems help researchers study atherosclerosis more effectively and open new therapeutic avenues.
Microfluidics: A New Frontier in Atherosclerosis Research
Microfluidic perfusion systems have emerged as a powerful alternative to traditional research models. By using small channels that mimic the architecture of blood vessels, microfluidic systems enable the long-term cultivation of vascular endothelial and epithelial cells under dynamic flow conditions, making them ideal for studying atherosclerosis. [2]
The ibidi Pump System, for example, is designed specifically for conducting flow assays under physiologically relevant conditions. This system enables researchers to apply controlled levels of shear stress to cultured cells, simulating the forces they would experience in vivo. By adjusting flow rates and patterns, scientists can recreate the various hemodynamic environments found in different regions of the circulatory system.
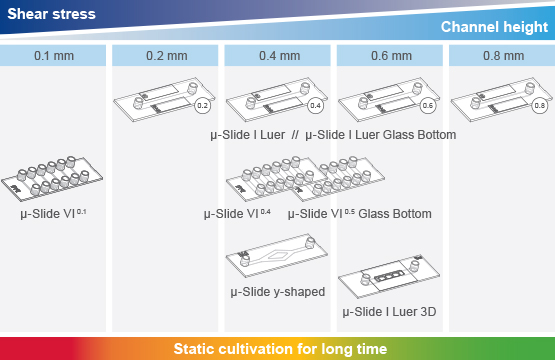
The Optimal Perfusion Set and Slide for Your Application
ibidi offers a variety of Channel Slides with different volumes and geometries, which can be paired with Perfusion Sets featuring various tube lengths and inner diameters. Each combination of a Perfusion Set and Channel Slide can achieve a specific shear stress range using the ibidi Pump System. Use the Perfusion Set and µ-Slide Selection Guide in the Product Details tab to select the optimal combination for your required shear stress.
ibidi Flow Calculator
Simple calculation tool
for your flow experiments
A major advantage of these systems is their ability to replicate both healthy and pathological flow conditions. In healthy vessels, endothelial cells exposed to laminar shear stress maintain anti-inflammatory properties, protecting against plaque formation. However, in disturbed flow areas, such as bifurcations, endothelial cells experience low or oscillatory shear stress, leading to dysfunction, inflammation, and early plaque development [3]. By simulating these conditions, microfluidic perfusion systems provide a powerful platform to study the cellular mechanisms behind atherosclerosis progression.
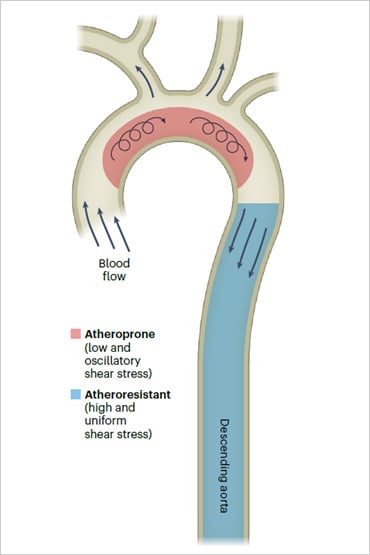
Impact of Vessel Geometry on Blood Flow Dynamics and Endothelial Cell Health
In straight segments of the vasculature (e.g., the descending aorta), blood flow is laminar, promoting endothelial cell (EC) health and resistance to atherosclerosis. In contrast, bifurcated or curved regions (e.g., the aortic arch) experience low and oscillatory blood flow patterns, which contribute to pathological changes in ECs, increasing their susceptibility to atherosclerosis. Microfluidic perfusion systems can replicate these varied flow conditions, providing insights into the mechanisms underlying vascular dysfunction and disease progression. The figure was reproduced under CC BY 4.0 from Aitken C et al., Nat. Cardiovasc. Res. 2:517–529, 2023.
Essential Readouts from Atherosclerotic Microfluidic Models
Microfluidic perfusion systems offer several key readouts essential for studying atherosclerosis, enabling researchers to investigate how endothelial cells (ECs) respond to various shear stress conditions over time. These systems simulate physiological flow environments, allowing for real-time observation of cellular behavior and responses that mirror in vivo conditions.
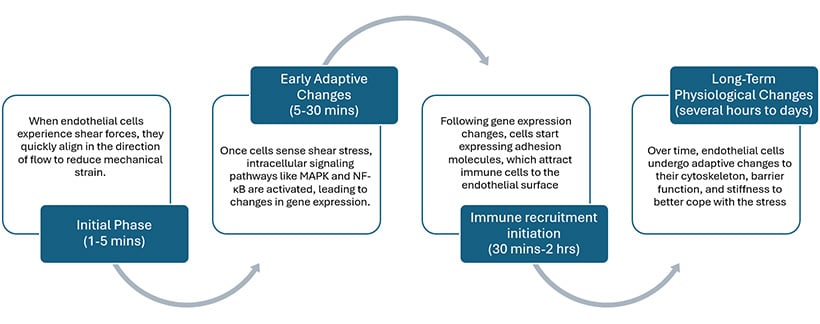
Shear Stress-Induced Cellular Changes in Endothelial Cells Over Time
Cell Morphology
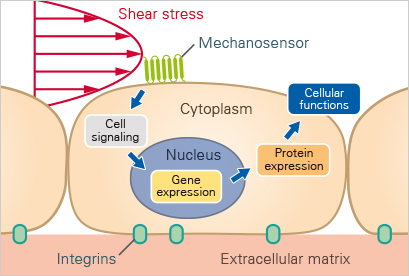
Under different flow conditions, endothelial cells exhibit distinct morphological changes essential for vascular health. In areas of laminar flow, cells align with the direction of flow, adopting an elongated shape. This alignment reduces mechanical stress and promotes optimal blood flow. In contrast, under oscillatory flow, cells lose their alignment and exhibit a more irregular shape. This morphological shift is associated with dysfunction and inflammation, disrupting normal signaling and potentially leading to conditions like atherosclerosis.
Gene Expression
Flow conditions significantly influence gene expression in endothelial cells. Under varying shear stress, endothelial cells may upregulate inflammatory genes, including cytokines and adhesion molecules, while also expressing genes that promote vascular health, such as those involved in nitric oxide production. [4]
Cell Adhesion and Inflammation
One of the earliest events in atherosclerosis is the adhesion of immune cells to the endothelial layer. Perfusion systems enable researchers to investigate how different flow environments influence the expression of adhesion molecules, like ICAM-1 and VCAM-1, and immune cell recruitment. This research provides insights into the mechanisms by which inflammation contributes to plaque formation and vascular disease progression.
Downstream effects of shear-stress induced mechanotransduction
The figure was reproduced under CC BY 4.0 from Chien S., Am J Physiol Heart Circ Physiol. 2007;292
Cell Physiology
Long-term physiological adaptations include cytoskeletal rearrangements, enhanced barrier function, and increased mechanical stiffness, which help cells cope with continuous shear stress. These structural changes contribute to cellular integrity, affecting the progression of atherosclerosis.
Applications in Drug Development and Therapy Testing
By simulating the conditions under which atherosclerosis develops, these systems provide a platform for testing the efficacy of potential treatments in a controlled environment. For example, researchers can use perfusion systems to test how different drug candidates affect endothelial cell function, plaque formation, and inflammation under realistic flow conditions [5]. This can accelerate the drug development process by providing early insights into how treatments might perform in vivo, reducing the need for animal testing and increasing the likelihood of success in clinical trials.
Additionally, perfusion systems can be used to study how existing therapies, such as statins or anti-inflammatory drugs, affect plaque progression and regression. By recreating the mechanical forces of blood flow, researchers can gain a better understanding of how these therapies work at the cellular level, leading to more targeted and effective treatments.
Case Study: Mimicking Atherosclerosis with the ibidi Pump System
The study titled "Endothelial Cannabinoid CB1 Receptor Deficiency Reduces Arterial Inflammation and Lipid Uptake in Response to Atheroprone Shear Stress" by Chen B, et al, [6] examines how marijuana-related signaling impacts cardiovascular health, specifically the role of endothelial CB1 receptors (CNR1) in atherosclerosis. The findings show that atheroprone shear stress increases CNR1 expression, leading to vascular dysfunction, inflammation, and lipid uptake, accelerating atherosclerotic lesion formation. Using the ibidi Pump System to simulate oscillatory shear stress in human aortic endothelial cells (HAoECs), the researchers revealed this stress elevates CNR1 expression. They found that endothelial CB1 deficiency is associated with reduced vascular inflammation and plaque buildup, suggesting that targeting peripheral CB1 receptors may provide a promising strategy for atherosclerosis and metabolic diseases without central side effects.
Overall, the findings underscore the importance of endothelial CB1 in linking shear stress to lipid metabolism and inflammation in atherosclerosis, highlighting it as a potential target for mitigating disease progression.
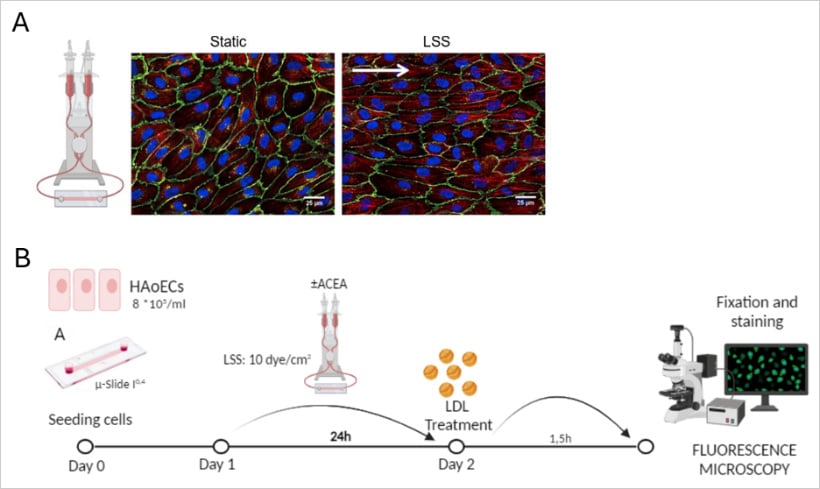
A: Schema of the perfusion chamber and the mechanism used to produce the shear stress
Representative immunofluorescence images of human umbilical vein endothelial cells (HUVEC) in static and Laminar Shear Stress (LSS) conditions.
B: Schematic of the workflow for LDL uptake experiments in HAoECs.
Cells were treated with ACEA (CB1 agonist) and exposed to 24 h of LSS before a 1.5-hour Dil-LDL incubation. The images were acquired to analyze Dil-LDL uptake.
Conclusion: The Future of Atherosclerosis Research
As cardiovascular research continues to advance, microfluidic systems are anticipated to play a crucial role in understanding the complexities of atherosclerosis. These systems allow for precise control over experimental conditions and support high-throughput analysis, enabling researchers to investigate a wide range of cellular responses simultaneously [2, 7]. Integrating advanced imaging techniques with microfluidics further enhances the ability to visualize cellular and molecular events in real time, allowing researchers to capture subtle changes in endothelial behavior as they occur [8]. Additionally, microfluidic platforms can be customized to include patient-derived cells, paving the way for personalized medicine approaches that take individual variability into account. This precision makes it possible to identify patient-specific factors contributing to atherosclerosis, tailoring therapies to the unique characteristics of each individual. In the future, the combination of microfluidic technology with AI-driven data analysis could accelerate the identification of novel drug targets and biomarkers, transforming the way cardiovascular diseases are diagnosed, treated, and managed. Ultimately, the ongoing development of microfluidic systems promises to bring a deeper understanding of vascular biology, pushing the boundaries of cardiovascular research and improving patient outcomes on a global scale.
References
- Hua, Y., et al., The Induction of Endothelial Autophagy and Its Role in the Development of Atherosclerosis. Frontiers in Cardiovascular Medicine, 2022. 9.
- Zheng, H., et al., Microfluidic-based cardiovascular systems for advanced study of atherosclerosis. Journal of Materials Chemistry B, 2024. 12(30): p. 7225-7245.
- Aitken, C., et al., Mechanisms of endothelial flow sensing. Nat Cardiovasc Res, 2023. 2(6): p. 517-529.
- Chien, S., Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. American Journal of Physiology-Heart and Circulatory Physiology, 2007. 292(3): p. H1209-H1224.
- Lysyy, T., et al., Ex vivo isolated human vessel perfusion system for the design and assessment of nanomedicines targeted to the endothelium. Bioengineering & Translational Medicine, 2020. 5(2): p. e10154.
- Chen, B., et al., Endothelial cannabinoid CB1 receptor deficiency reduces arterial inflammation and lipid uptake in response to atheroprone shear stress. bioRxiv, 2024: p. 2024.05.15.594375.
- De Stefano, P., E. Bianchi, and G. Dubini, The impact of microfluidics in high-throughput drug-screening applications. Biomicrofluidics, 2022. 16(3).
- Maringanti, R., et al., Atherosclerosis on a Chip: A 3-Dimensional Microfluidic Model of Early Arterial Events in Human Plaques. Arteriosclerosis, Thrombosis, and Vascular Biology. 0(0).
 (4)
(4)  (0)
(0)