From Tumor Microenvironment to Microscope: Capturing Cancer in Motion
ibidi Blog | August 20, 2025 | Abhishek Derle, ibidi GmbH
What if you could watch cancer unfold in real time, seeing tumor cells divide, migrate, and respond to stress right under the microscope. Imagine if you could recreate a miniature, living version of the tumor microenvironment (TME) in your lab and track how cancer behaves in its natural habitat.
Modern oncology research is making this possible. Scientists are now stepping inside the tumor’s world, capturing its life in motion through live cell imaging. By combining tumor cells with fibroblasts, immune cells, and extracellular matrix within engineered 3D scaffolds, they can recreate miniature tumor ecosystems [2].
Microfluidic 3D models and perfused culture systems make it possible to sustain these tumors in realistic conditions for hours or even days. Meanwhile, high‑resolution live cell imaging reveals cancer’s story as it happens—how it grows, invades, interacts with immune cells, and responds to therapy in real time.
Why the Tumor Microenvironment Holds the Key
Cancer thrives in complexity. It does not grow in isolation but as part of a bustling neighborhood of cells and signals. This surrounding ecosystem fuels growth, enables metastasis, and shields the tumor from treatment. Studying this microenvironment, rather than the tumor in isolation, provides researchers a far more realistic understanding of how cancer behaves.
To truly understand it, researchers need to move beyond oversimplified 2D cultures and recreate the tumor microenvironment in the lab. This requires modeling its most critical features:
Hypoxia
As tumors grow faster than their blood supply, pockets of oxygen deprivation form, sometimes dropping below 1%. Low oxygen levels stabilize HIF‑1α, triggering angiogenesis, metabolic changes, and epithelial-to-mesenchymal transition (EMT). It also makes tumors harder to treat, which is why faithfully replicating hypoxia in vitro is so valuable [3].
Cell–cell and Cell–matrix Interactions
Tumor cells are in constant conversation with surrounding stromal and immune cells through integrins, cadherins, and soluble factors. These signals drive invasion, immune evasion, and therapy resistance. Embedding multiple cell types in 3D models helps capture these interactions as they naturally occur.
Immune Infiltration and Suppression
Macrophages, regulatory T cells, and other immune players can either attack the tumor or help it hide. Mimicking these interactions in vitro is essential for studying how tumors evade immune surveillance and for improving immunotherapies, such as checkpoint inhibitors [4].
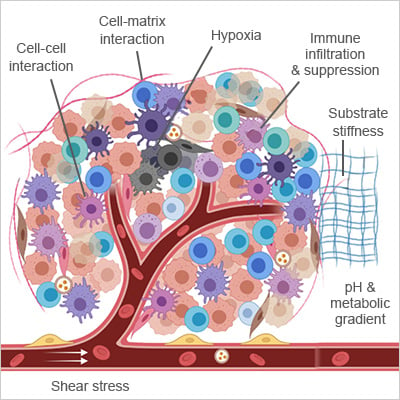
Key components of tumor microenvironment (TME).
Adapted from [1]
Shear Stress and Interstitial Flow
The physical forces created by fluid movement influence how cancer cells migrate, how vessels form, and how metastasis begins [5]. Perfused 3D models allow scientists to study these mechanics under conditions that resemble the body.
Nutrient and Metabolite Gradients
Uneven blood supply and high metabolic demand create steep gradients in glucose, amino acids, and pH. These pressures promptcancer cells to adapt, triggering autophagy, metabolic rewiring, and the selection of aggressive clones, which drive heterogeneity and drug resistance [6].
By recreating these conditions in 3D culture models, researchers can study tumors in a way that is far closer to reality.
Live Cell Imaging: Watching Tumors Think
Cancer is dynamic. Tumor cells are constantly on the move, invading surrounding tissue, forming new vessels, and engaging in a constant battle with the immune system. Live cell imaging transforms these invisible processes into a real-time story, allowing researchers to witness how tumors behave, adapt, and respond to therapy as it happens.
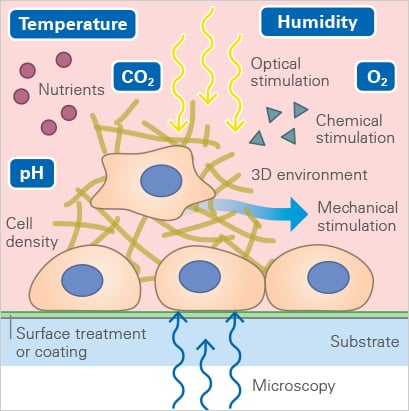
Capturing these events over hours or even days is not as simple as leaving cells under a microscope. Even minor changes in temperature, humidity, or gas composition can stress cells, alter their behavior, or create imaging artifacts. Maintaining a stable, physiological environment is crucial for accurately reflecting how tumors behave in the body.
This is where stage-top incubation becomes essential. Systems like the ibidi Stage Top Incubator keep cells in a physiological environment right on the microscope. Temperature, humidity, and gas levels remain tightly controlled, with adjustable oxygen for hypoxia studies. A heated lid prevents condensation, and active humidity control prevents evaporation, so cells stay healthy and the medium remains stable throughout the experiment. With these conditions locked in, researchers can capture high‑resolution, artifact‑free images of 3D tumor models over time.
Tumor‑on‑a‑Chip: Reconstructing the Real
Live cell imaging becomes even more powerful when combined with microfluidic 3D models, often referred to as tumor-on-a-chip systems. These miniature platforms replicate the complexity of a living tumor far more accurately than static cultures by incorporating perfusable channels, immune components, and extracellular matrix [7].
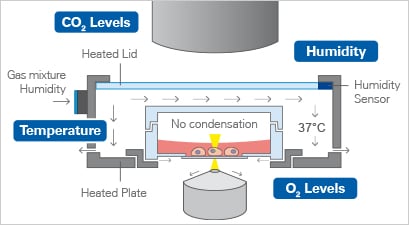
A stage-top incubator keeps cells healthy by controlling temperature, gas, and humidity for high-quality live imaging
Examples of such platforms include the µ-Slide Spheroid Perfusion, which supports 3D spheroid culture under continuous, low-shear perfusion on a Bioinert surface, ensuring that spheroids remain free-floating, viable, and easily imaged. For cells embedded in 3D matrices like collagen, the µ-Slide III 3D Perfusion enables long-term experiments with six wells connected via perfusable channels, making it perfect for observing invasion, therapeutic response, or immune infiltration. Both slides connect seamlessly to the ibidi Pump System, which delivers controlled perfusion and stable shear stress, recreating conditions like hypoxia, nutrient gradients, and interstitial flow with high precision. For high‑throughput work, the µ‑Plate 96 Well 3D provides a cost‑effective solution for generating uniform spheroids across dozens of wells, ideal for drug screening and longitudinal studies.
By combining live cell imaging with tumor-on-a-chip systems, researchers can visualize the tumor microenvironment as it truly functions, bringing in vitro cancer studies closer than ever to the complexity of the human body.
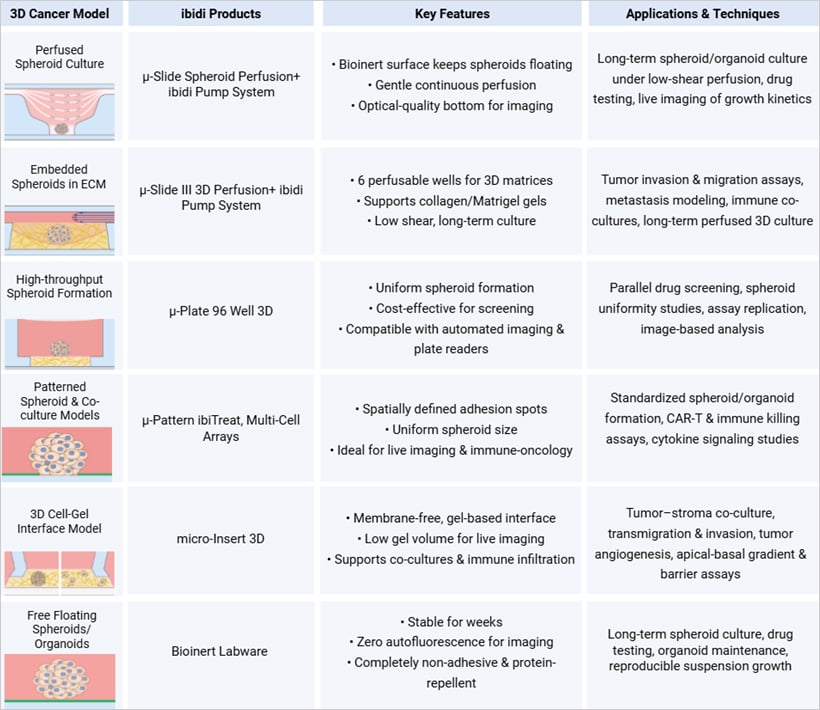
Overview of ibidi 3D culture solutions for tumor microenvironment modeling and imaging.
Patterning the Tumor: Shaping Precision in 3D
One challenge in 3D tumor research is variability. Spheroids naturally form in different sizes and shapes, which complicates comparisons and reproducibility. Micropatterning technologies, such as ibidi’s Micropatterned Labware, address this issue by guiding cells to adhere only to predefined spots, resulting in uniform, size-controlled spheroids that are ideal for imaging.
This precision extends to co-culture models. By arranging cancer, immune, and stromal cells in reproducible patterns, researchers can generate highly controlled tumor-immune interaction studies. Using live cell imaging, researchers can track processes such as CAR-T cell targeting, immune synapse formation, and cytokine signaling in real-time [8]. Patterned models not only enhance reproducibility but also provide clear, visual insights into how cells coordinate, compete, or attack within a tumor-like microenvironment.
Single Cells in a 2D Environment
Time lapse microscopy of a CAR-T cell killing assay with RCC-26 tumor cells and JB4 T cells on a single cell pattern (µ-Slide VI 0.4, 20 µm squares, 120 µm distance, rectangular). Data were analyzed using FastTrack AI by MetaVi Labs.
Multi-Cell Spots in a 3D Collagen Matrix
RCC-26 cancer cells immobilized on multi-cell pads (µ-Slide VI 0.4, 200 µm circles, 600 µm distance, hexagonal). Effector T cells applied in a collagen I matrix (Collagen Type I, Rat Tail) induce apoptotic body formation of cancer cells.
Final Thoughts: Recreate to Reveal
To truly grasp the complexities of cancer, we must delve into the distinctive environment it generates and understand how it shapes the disease itself. Modern 3D models and live imaging are shifting cancer research from static snapshots to living, dynamic systems that capture the disease in motion.
By recreating the tumor microenvironment with hypoxia, flow, immune interactions, and nutrient gradients, researchers can observe the processes that drive cancer progression as they unfold. This approach not only enables the visualization of tumor dynamics but also improves the predictive power of preclinical models, thereby helping to bridge the gap between lab research and clinical application.
References
- Sheth, M. and L. Esfandiari, Bioelectric Dysregulation in Cancer Initiation, Promotion, and Progression. Frontiers in Oncology, 2022. Volume 12 - 2022.
- Anderson, N.M. and M.C. Simon, The tumor microenvironment. Curr Biol, 2020. 30(16): p. R921-r925.
- Chen, Z., et al., Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduction and Targeted Therapy, 2023. 8(1): p. 70.
- Du, W., et al., Extracellular matrix remodeling in the tumor immunity. Frontiers in Immunology, 2024. Volume 14 - 2023.
- Rahman, Z., et al., Interstitial flow potentiates TGF-β/Smad-signaling activity in lung cancer spheroids in a 3D-microfluidic chip. Lab Chip, 2024. 24(3): p. 422-433.
- Guerrero-López, P., et al., 2D versus 3D tumor-on-chip models to study the impact of tumor organization on metabolic patterns in vitro. Scientific Reports, 2025. 15(1): p. 19506.
- Jouybar, M., et al., Cancer-on-chip models for metastasis: importance of the tumor microenvironment. Trends in Biotechnology, 2024. 42(4): p. 431-448.
- Wang, X., et al., Dynamic Profiling of Antitumor Activity of CAR T Cells Using Micropatterned Tumor Arrays. Advanced Science, 2019. 6(23): p. 1901829.
 (1)
(1)  (0)
(0)



