How to Study Endothelial Cells of the Cardiovascular
System in Vitro
ibidi Blog | July 18, 2023 | Elisabeth Kugler, Zeeks – Art for Geeks Ltd (for ibidi)
Endothelial cells (ECs) are specialized cells that line the inner surface of blood vessels, forming a continuous single-cell layer. They are, therefore, part of the blood vessel wall and play a crucial role in controlling the exchange of substances between the bloodstream and surrounding tissues [1]. ECs are further sub-specialized based on their location in the circulatory system, such as arteries, veins, capillaries, and lymphatics. In this blog article, we explore the functions of ECs, focusing on ECs of the cardiovascular system. Specifically, we will examine how to study ECs in vitro in angiogenesis, barrier formation, under flow, and inflammation.
ECs in Angiogenesis
Angiogenesis is the process of the growth of new blood vessels. Thus, it plays a role in developmental biology, wound healing, as well as tumor growth (Fig. 1). Angiogenesis itself is made of various steps, including:
- EC proliferation
- directed migration (often via chemotaxis)
- tube formation and lumenization
- maturation: fusion, remodeling, recruitment of secondary cells (e.g., pericytes and vascular smooth muscle cells)
Together, these steps contribute to the overall angiogenic process, namely forming new vessels from existing ones [2]. Undoubtedly, angiogenesis is highly intricate. Gaining a comprehensive understanding of the overall process requires us to examine the individual components. Employing in vitro assays enables scientists to concentrate on specific components of the angiogenesis process, facilitating a more controlled and detailed analysis.
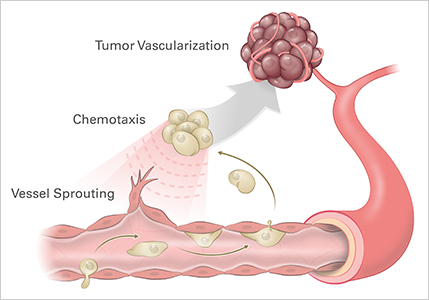
Figure 1. Studying angiogenic processes, such as vessel sprouting and chemotaxis, is critical to understanding tumor vascularization.
Application Example 1: Elucidating the Role of Gap Junctions in EC Migration
This study by Mannell et al. 2021 examined the role of gap junction protein connexin 43 (Cx43) in EC migration and angiogenesis. The team showed that the knock-down of Cx43 by siRNA in human microvascular endothelial cells (HMEC) reduced cell migration (Fig. 2). Mechanistically, the group showed that the Cx43 function was mediated via interaction with the tyrosine phosphatase SHP-2 [3].
To investigate CX43 in EC migration, ibidi Culture-Inserts were placed in the µ-Slide 8 Well, as the study previously described, to assess migration speed and directionality [3].
Together, the group showed that EC migration and angiogenesis require Cx43 and that this is mediated by SHP-2.
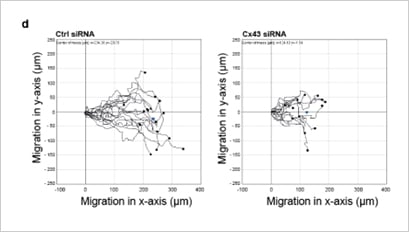
Figure 2. Representative single-cell traces of migrating HMEC show a reduction of migration upon knock-down of Cx43 siRNA. The figure was reproduced under CC BY 4.0 from Mannell et al. 2021 [3] Figure 1D.
Find out more about Angiogenesis.
ECs in Barrier Formation
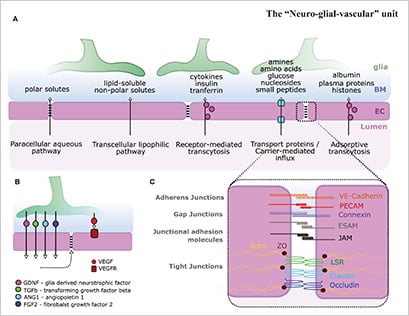
The single-cell layer of ECs in blood vessels is critical in its function to form barriers between blood and the surrounding tissue, most famously the blood-brain barrier (BBB). ECs actively regulate compound transport via several pathways, including paracellular aqueous pathway, transcellular lipophilic pathway, receptor-mediated transcytosis, carrier-mediated influx, and adsorptive transcytosis (Fig. 3).
Understanding the functions and mechanism of EC barriers is a highly studied research area due to its importance in drug delivery, pharmacology, toxicology, and oncology.
Figure 3. ECs (magenta) play a critical role in barrier formation. Figure was reproduced under CC BY 4.0 license from Kugler et al., 2021 [5]. (Click to enlarge)
Application Example 2: Establishing In Vitro Models of The Blood-Brain-Barrier
In a study conducted by Choublier et al. in 2021 [6], the experimental challenges associated with studying the BBB were addressed. The BBB presents difficulties due to its location in the brain, as well as the need for a constant, laminar, and homogeneous blood flow. To address these challenges, the researchers developed a robust, low-cost device where the upper channel was connected to the ibidi Pump System to establish unidirectional recirculation of the culture medium for four days to mimic physiological conditions.
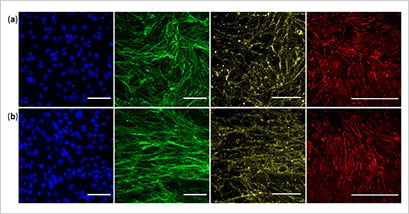
Choublier and colleagues showed that the device was suitable for evaluating barrier function (Fig. 4) and investigating drug transport across the BBB. Moreover, the ibidi Pump System and µ-Slide have the potential to evaluate and replicate barriers found in human cell types like the intestines or kidneys.
Establishing a system with regulated flow allows the study of ECs under in vivo-like conditions, which mimic true physiologically better than a static system.
Figure 4. Cells cultured for seven days in static conditions (a) and under static flow (b), showing endothelial monolayer specialization. Blue – nuclei, green - F-actin, yellow - β-catenin adherent junction, and red - ZO-1 tight junction. Reproduced under CC BY 4.0 Figure 4 from Choublier et al. in 2021 [6]. (Click to enlarge)
ECs Under Flow
Shear stress created by blood flow has a direct impact on EC cell polarization, protein expression, and morphology (Fig. 5). While one major research area is to understand how static flow contributes to physiological ECs, another research area is to examine how perturbed flow regimes contribute to diseases such as atherosclerosis.
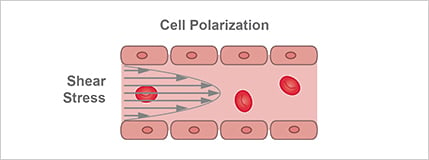
Figure 5. Shear stress created by blood flow directly impacts cell polarization, protein expression, and morphology.
Application Example 3: Deciphering the Role of Mitochondria in EC Health
This work by Hong et al. 2022 [7] examines the role of mitochondria in maintaining EC homeostasis and health. The findings demonstrated that mitochondrial fragmentation is increased in regions exposed to disturbed flow, while elongated mitochondria are predominant in regions of unidirectional flow. This suggests that flow patterns have a profound impact on mitochondrial fusion/fission events, influencing the proinflammatory and metabolic states of ECs. The researchers employed the ibidi Pump System to study these flow pattern-dependent dynamics.
Together, this study showed that flow has a critical impact on EC health and that this is in part due to mitochondrial changes.
Find out more about Cell Culture Under Flow.
ECs in Inflammation
Systemic inflammation has a direct effect on ECs as well as how ECs interact with other cells. Generally, the barriers formed by ECs become leakier [8], and processes such as immune cell rolling, chemotaxis, and trans-endothelial migration are elevated during inflammation (Fig. 6).
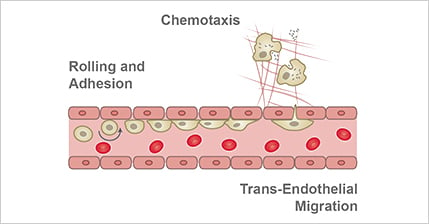
Figure 6. Inflammatory processes impact ECs, as well as how ECs interact with other cells. For example, processes such as immune cell rolling, chemotaxis, and trans-endothelial migration are elevated during inflammation.
Application Example 4: Studying Factors Contributing to Atherosclerosis
In a study by Forde et al. in 2020 [9], the effects of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on human aortic endothelial cells (HAECs) under pro-atherogenic conditions were studied. The results showed that TRAIL shifted the gene expression towards an antioxidant profile in HAECs exposed to oscillatory shear stress, thus being vasoprotective. Additionally, TRAIL significantly reduced reactive oxygen species (ROS) formation in HAECs exposed to both TNF-α and hyperglycaemia. These findings suggest that TRAIL has atheroprotective effects on the endothelium by reducing oxidative stress.
This study used the ibidi Pump System and an ibidi Channel Slide to establish a state-of-the-art culture model of pro-atherogenic oscillatory shear stress, known to promote atheroma formation [10].
Find out more about Immunology.
In this blog, we explored ECs in the cardiovascular system and the significance of studying ECs in vitro. We aimed to understand the role of ECs in different physiological contexts, which allowed us to appreciate that not only the ECs specification and status are important, but also their environment.
By conducting research in controlled conditions, in vitro studies provide valuable insights into processes that would be challenging or impossible to explore through in vivo experiments alone. Through examining various mechanisms and processes such as angiogenesis, barrier formation, under flow, and inflammation, we underscored the value of in vitro studies.
References
[1] Krüger-Genge A, Blocki A, Franke RP, Jung F. Vascular Endothelial Cell Biology: An Update. Int J Mol Sci. 2019 Sep 7;20(18):4411. doi: 10.3390/ijms20184411.
Read article
[2] Adair TH, Montani JP. Angiogenesis. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Chapter 1, Overview of Angiogenesis.
Read book chapter
[3] Mannell H, Kameritsch P, Beck H, Pfeifer A, Pohl U, Pogoda K. Cx43 Promotes Endothelial Cell Migration and Angiogenesis via the Tyrosine Phosphatase SHP-2. Int J Mol Sci. 2021 Dec 28;23(1):294. doi: 10.3390/ijms23010294.
Read article
[4] Kameritsch P, Kiemer F, Mannell H, Beck H, Pohl U, Pogoda K. PKA negatively modulates the migration enhancing effect of Connexin 43. Biochim Biophys Acta Mol Cell Res. 2019 May;1866(5):828-838. doi: 10.1016/j.bbamcr.2019.02.001.
Read article
[5] Kugler EC, Greenwood J, MacDonald RB. The "Neuro-Glial-Vascular" Unit: The Role of Glia in Neurovascular Unit Formation and Dysfunction. Front Cell Dev Biol. 2021 Sep 27;9:732820. doi: 10.3389/fcell.2021.732820.
Read article
[6] Choublier N, Müller Y, Gomez Baisac L, Laedermann J, de Rham C, Declèves X, Roux A. Blood–Brain Barrier Dynamic Device with Uniform Shear Stress Distribution for Microscopy and Permeability Measurements. Appl. Sci. 2021 Nov; 11(12):5584. doi.org/10.3390/app11125584
Read article
[7] Hong SG, Shin J, Choi SY, Powers JC, Meister BM, Sayoc J, Son JS, Tierney R, Recchia FA, Brown MD, Yang X, Park JY. Flow pattern-dependent mitochondrial dynamics regulates the metabolic profile and inflammatory state of endothelial cells. JCI Insight. 2022 Sep 22;7(18):e159286. doi: 10.1172/jci.insight.159286.
Read article
[8] Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013 Dec;19(12):1584-96. doi: 10.1038/nm.3407.
Read article
[9] Forde H, Harper E, Rochfort KD, Wallace RG, Davenport C, Smith D, Cummins PM. TRAIL inhibits oxidative stress in human aortic endothelial cells exposed to pro-inflammatory stimuli. Physiol Rep. 2020 Oct;8(20):e14612. doi: 10.14814/phy2.14612.
Read article
[10] Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009 Jan;6(1):16-26. doi: 10.1038/ncpcardio1397.
Read article
 (3)
(3)
 (0)
(0)