The Art and Science of 3D Cell Culture Through Microscopy
ibidi Blog | April 3, 2024 | Abhishek Derle, ibidi GmbH
Welcome to our exploration of the transformative world of 3D cell culture, a technique reshaping the biomedical research landscape. For a long time, scientists have relied on flat 2D cell models to study cellular and disease mechanisms due to their simplicity and cost-effectiveness. However, in recent years, 3D cell cultures have surged in popularity because of their ability to mimic in vivo tissue environments more accurately. If you think about it, cells rarely exist within our bodies as isolated monolayers. Therefore, studying 3D cell culture techniques offers researchers a powerful tool to explore cell behavior in a more relevant setting. In vitro, this can be achieved by either suspending cells on a non-adhesive surface or embedding them within a matrix that mimics the extracellular matrix, facilitating growth in all dimensions.
In this blog article, we’ll showcase captivating microscopy images that blend the artistry and scientific significance of 3D cell culture. These images, published in ibidi’s annual calendar through the photo contest, offer a unique glimpse into the beauty and complexity of cellular structures and interactions. From embryonic stem cell aggregates to intestinal spheroids to breast cancer organoids, these microscopy images will highlight diverse 3D cell cultures, providing insights into their respective fields of study. So, let's dive in!
Read on and learn more about Matrices for 3D Culture or different 3D Cell Culture Assays.
Do you have a stunning microscopy image that you would like to showcase in the next ibidi calendar? Then enter your image in the ibidi Photo Contest today!
Mouse Small Intestine Organoid
This magnificent image displays a 3D culture of a mouse small intestine organoid focused on assessing the IL-22 cytokine's impact on antimicrobial expression, notably the bactericidal protein Resistin-like molecule (RELM) β expression in the gastrointestinal tract. The organoids were cultured in Matrigel® drops within a pre-warmed ibidi µ-Slide 8 Well high, followed by immunofluorescence staining. They were stained for the antimicrobial protein RELMβ (green), the secretory cell marker Ulex Europaeus Agglutinin I (UEA I, red), β-catenin (purple), and the nuclear marker DAPI (blue). Confocal microscopy imaging was performed using a Zeiss LSM 880 confocal microscope with a 20x objective lens.
This study helped to closely recapitulate and mimic the in vivo intestinal epithelium, providing valuable insights into the effects of IL-22 on antimicrobial expression and the crosstalk between immune cell-derived cytokines and the intestinal epithelium.
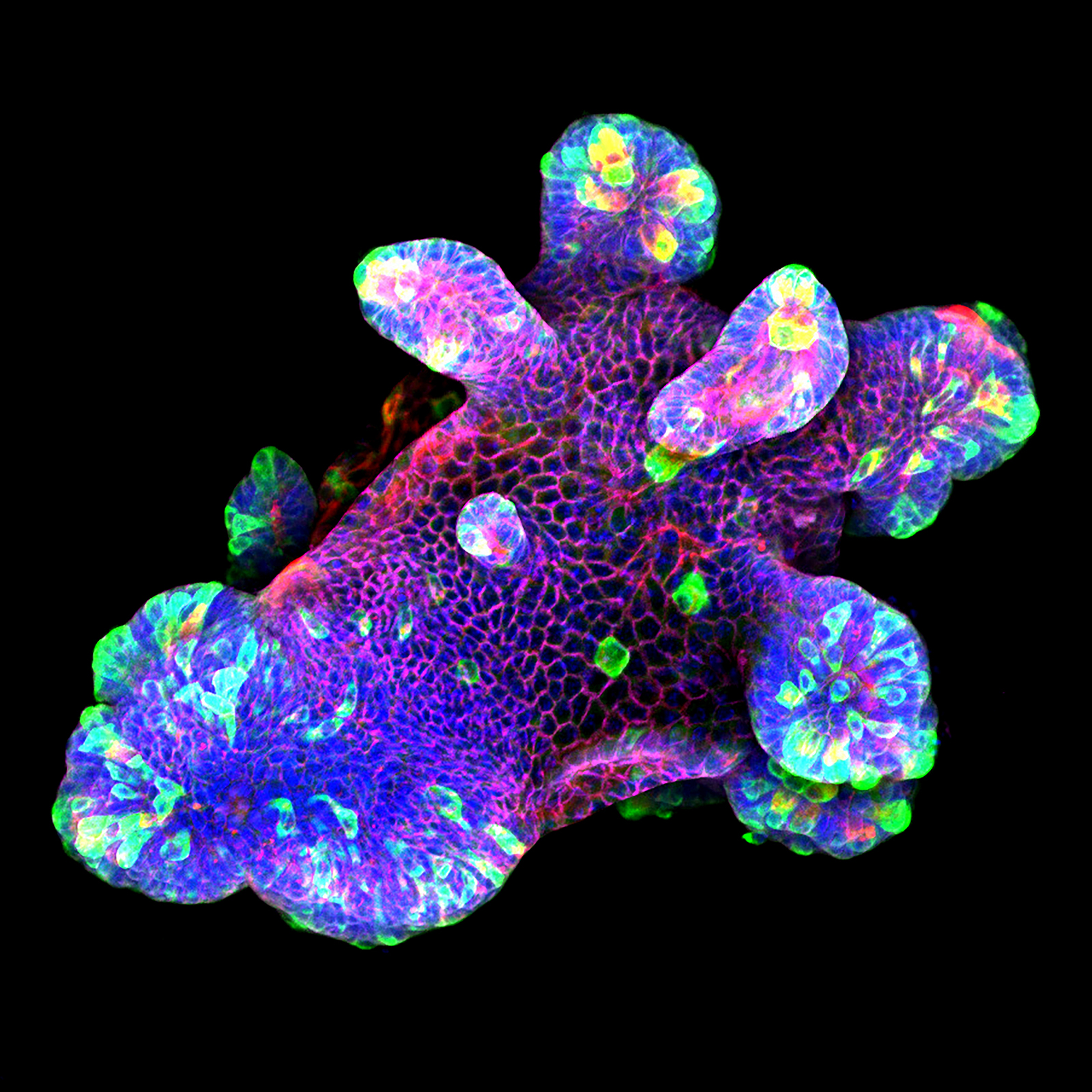
Scientific artwork by: Naveen Parmar from Department of Pathology, University of Oslo, Norway.
Human Breast Cancer Organoid
This image captures the complexity of a human breast cancer organoid, cultivated from a patient's tumor biopsy. The research focuses on creating a tumor microenvironment within the organoid that closely simulates drug interactions, aiming for advancements in personalized treatments.
Following the procurement of the tumor sample, it was meticulously dissected and subjected to enzymatic digestion. The processed tissue was then cultured within a supportive Matrigel matrix, fostering the growth of organoids.
These organoids undergo immunofluorescence staining to reveal key cellular markers: Ki67 in magenta, indicating proliferation; F-actin in green, outlining the cytoskeleton; and nuclei stained in blue. The organoid was cultured and imaged in a µ-Dish 35 mm, high Glass Bottom. The image was taken using ANDOR Dragonfly High Speed confocal microscope system with a 25x objective.
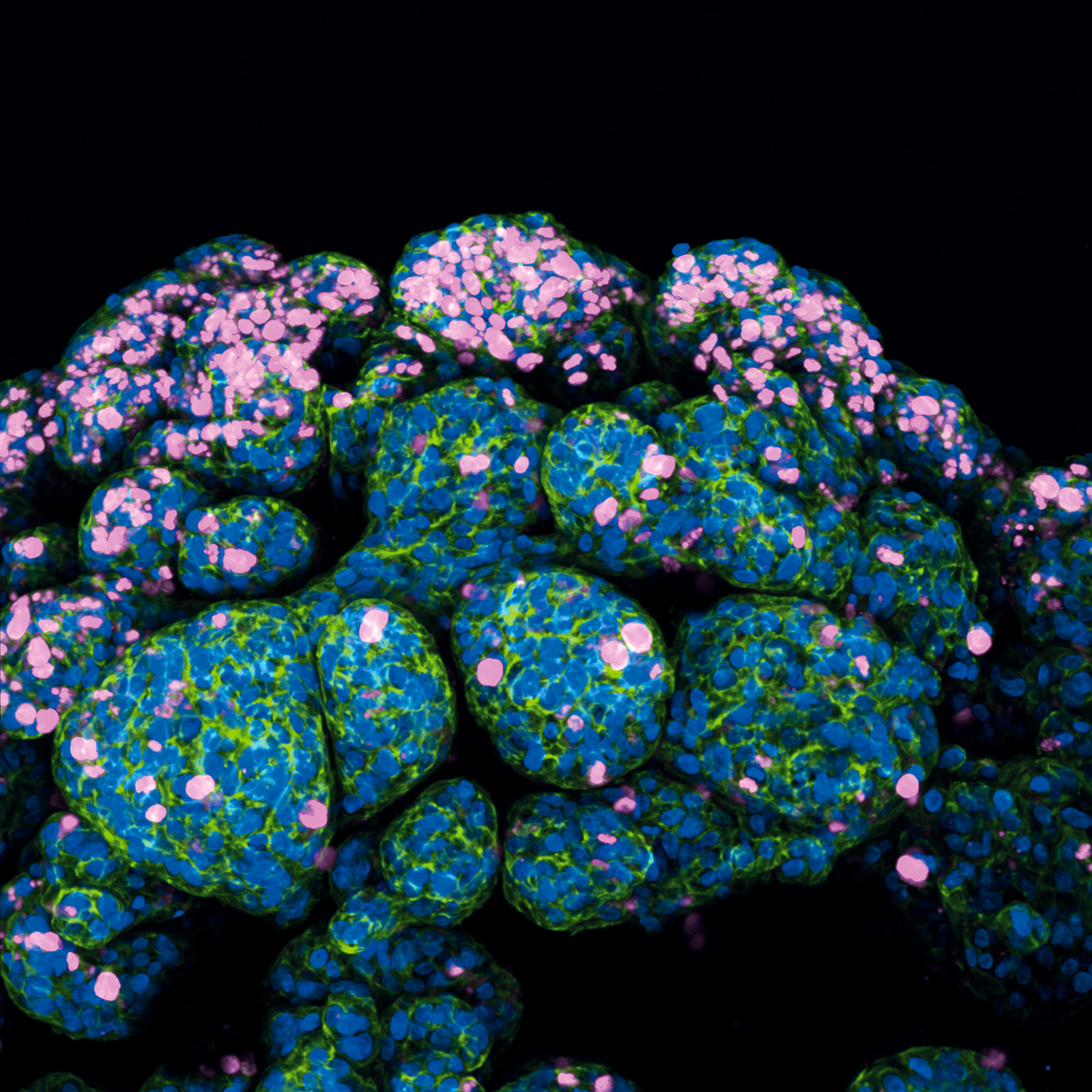
Scientific artwork by: Kai-Wen Kan from China Medical University Hospital, Taiwan.
3D Aggregates of Murine Embryonic Stem Cells
This image showcases three gastruloids, 3D aggregates of murine embryonic stem cells, just before axis extension, mimicking the separation of germ layer fates from pluripotent cells, as in embryos. The study investigates the spatial organization of cell fates and their influence on neighbors, analyzing effects at the tissue and single-cell levels in 3D.
Samples were prepared using strategic multiplexing of gene markers, including Sox2 (blue), Brachyury (T, green), Tbx6 (red), and LaminB1 (gray), highlighting the spatial arrangement and differentiation order of these transcription factors in neuromesodermal progenitors (NMPs). Subsequently, the samples underwent dehydration in increasing water:methanol dilutions to mount them in BABB, facilitating deep tissue imaging. This image serves as a visual demonstration of cell fate patterns and overall gastruloid morphology, captured using a Leica Sp8 confocal microscope with a 40x oil objective and the Leica LIGHTNING system on a µ-Slide 8 Well high.
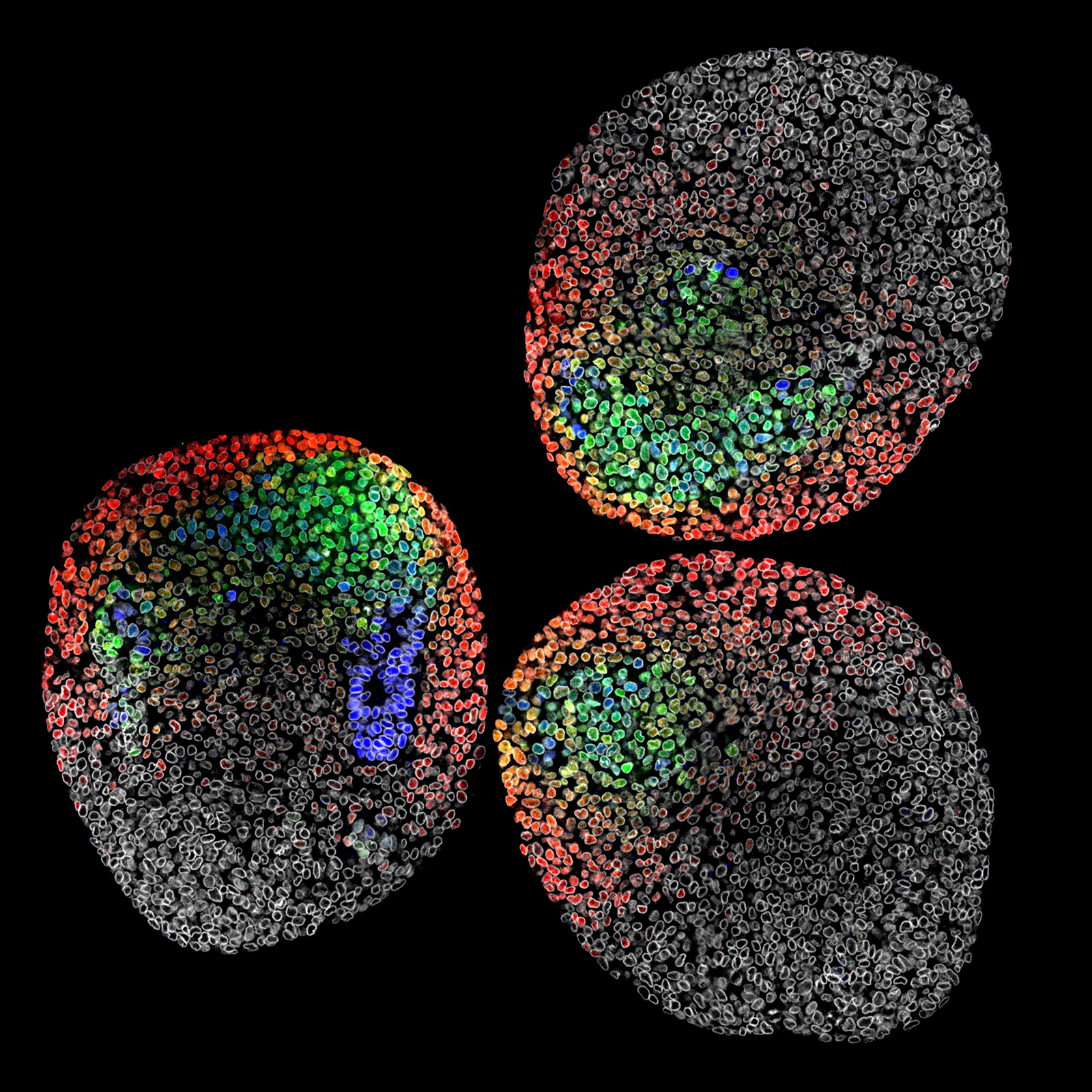
Scientific artwork by: Matthew French from University of Edinburgh, United Kingdom.
Murine Ileum Organoid
This image depicts a 3D culture of a murine ileum organoid, meticulously prepared in an ibidi µ-Slide 8 Well high coated with Matrigel®. The research aimed to analyze host-pathogen interactions, specifically focusing on human-restricted pathogens like typhoidal Salmonella.
The use of 3D organoid imaging facilitated the examination of the invasion and intracellular lifestyle of typhoidal Salmonella within a complex tissue environment. To visualize the organoid's details, staining techniques were applied: Phalloidin (green) for F-actin, CellMask (red) for the plasma membrane, and DAPI (blue) for nuclei. Imaging was performed with a Zeiss Cell Observer confocal microscope at 40x magnification. To achieve reasonable resolution and imaging dept, organoids were seeded in cold µ-Slide 8 Well high chamber slides to prevent Matrigel solidification, ensuring they settled near the coverslip bottom to optimize image resolution.
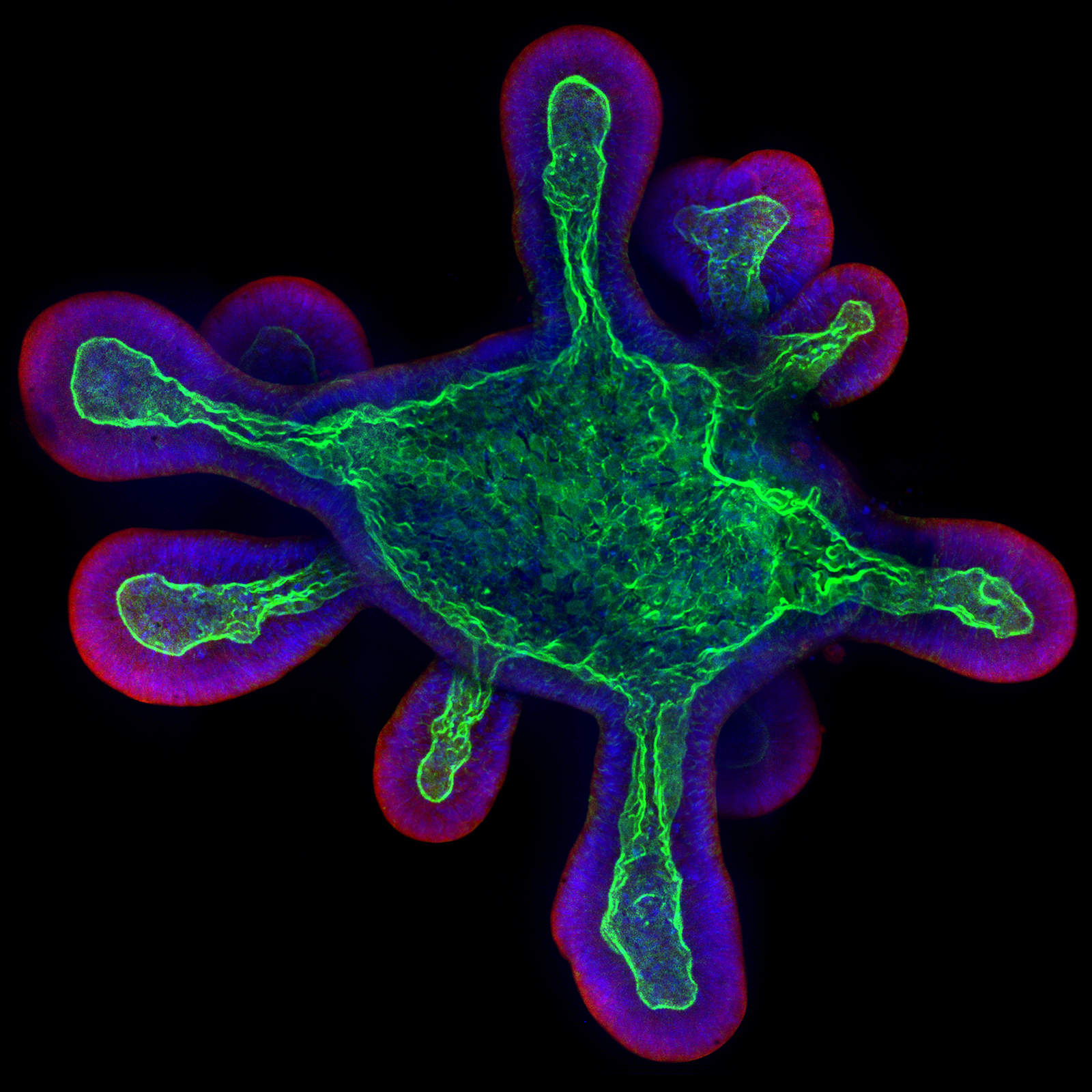
Scientific artwork by: Felix Scharte from MRC Laboratory of Molecular Biology, Cambridge, United Kingdom.
Human Intestinal Fibroblasts and Endothelial Cells Spheroid
This image illustrates a composite spheroid model integrating human intestinal fibroblasts and endothelial cells within a fibrin hydrogel. The focus of this research was to explore vascularization strategies within intestinal organoids. The model, known as Vascularization Units (VUs), combines human endothelial progenitor cells with intestinal fibroblasts cultured in a fibrin gel on an ibidi µ-Slide 4 Well for three days. The staining process highlighted CD31 in yellow to mark endothelial cells, vimentin in red for fibroblasts, and DAPI in cyan to identify DNA, utilizing a Leica TCS SP5 II confocal microscope with a 10x objective lens for imaging.
A significant observation was the overgrowth of fibroblasts, impeding proper vessel formation within the spheroid. To mitigate this, the research protocol was adapted by inverting the ibidi µ-Slide during the gelification process . This adjustment successfully prevented the spheroid from sinking and attaching to the bottom of the well, facilitating more effective vascularization and enhancing the overall research outcomes.
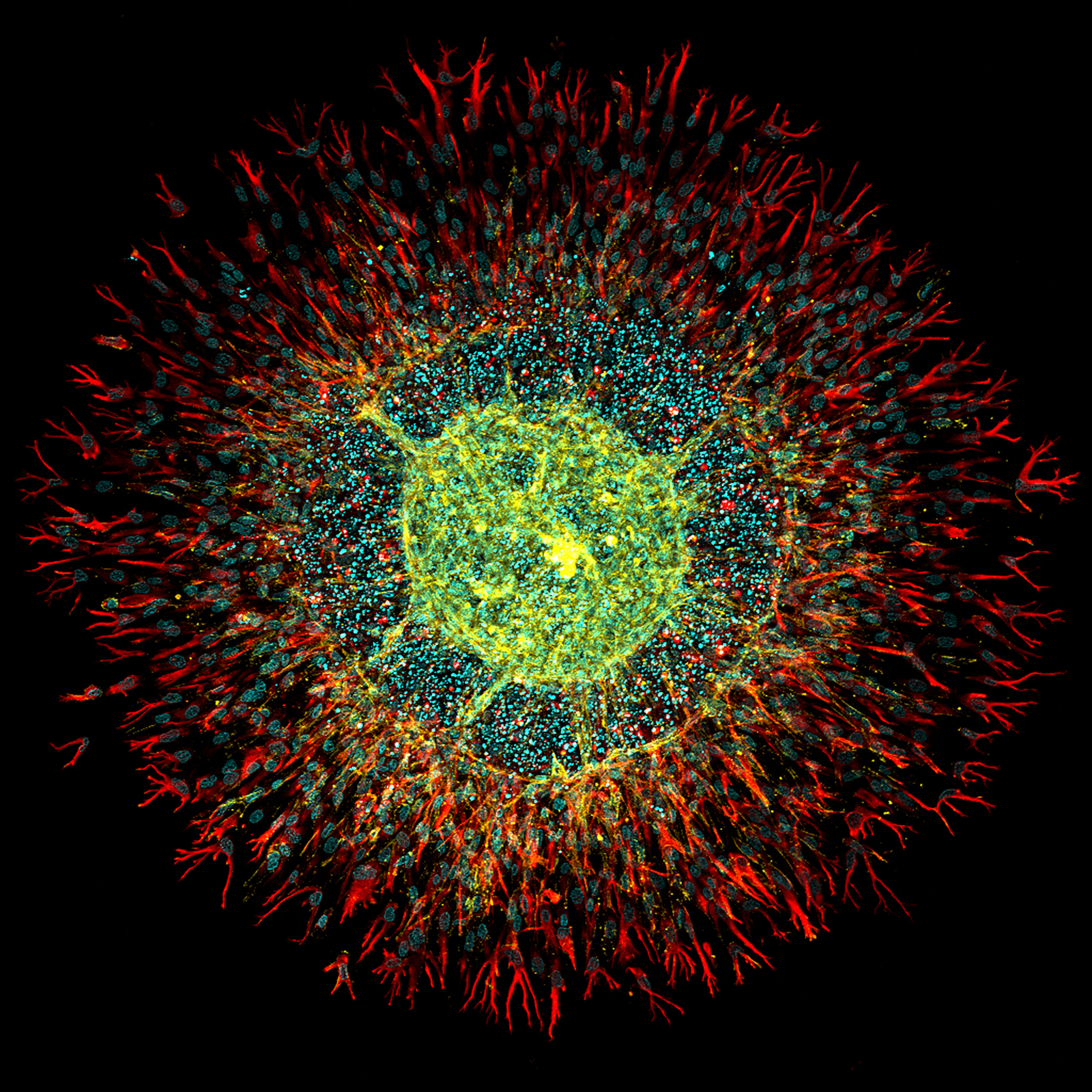
Scientific artwork by: Henrique Nogueira Pinto from Dept. Molecular Cell Biology & Immunology, Amsterdam UMC, The Netherlands
Discover more stunning cell images in our CellArt Gallery and Instagram Channel.
For more information on spheroids, organoids, and 3D Cell Culture, visit our 3D Cell Culture Application Site or download our Application Guide 3D Cell Culture.
 (1)
(1)
 (0)
(0)





