Fluorescence Recovery After Photobleaching (FRAP)
Applications:
FRAP is a fluorescence microscopy method for studying the mobility of fluorescently-labeled molecules in living cells. It is applied for the analysis of molecule diffusion within the cell, fluidity of bio membranes, and protein binding.
Principle:
FRAP can be conducted using a modern confocal microscope. It needs fluorescent labeling of the molecule of interest, by a GFP-tagged antibody, for example. A typical FRAP experiment involves three distinct phases. First, the initial fluorescence is measured in the region of interest. Next, the fluorescent molecules are photobleached within a selected area. This is done by focusing the laser beam onto the respective area, leading to intense illumination and extinction of the fluorophores. As a result, a dark area in the otherwise fluorescent sample can be observed. Finally, fluorescent molecules from the surroundings can diffuse through the sample and exchange the photobleached molecules with intact ones. This fluorescence recovery is measured over time.
Since many factors can influence the recovery kinetics, the FRAP experiments have to be planned well and the resulting data must be analyzed carefully. It is then possible to obtain the diffusion coefficient and molecule mobility parameters using mathematical modelling.
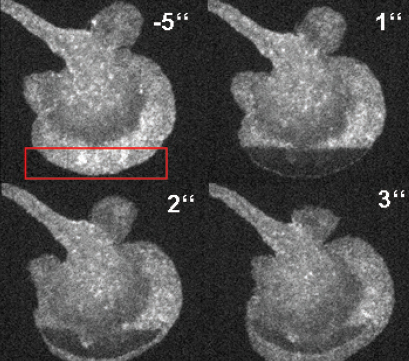
FRAP-based visualization of the F-actin diffusion in a primary dendritic cell.
H.C. Ishikawa-Ankerhold, R. Ankerhold, G.P.C. Drummen. Advanced Fluorescence Microscopy Techniques—FRAP, FLIP, FLAP, FRET and FLIM. Molecules, 2012, 10.3390/molecules17044047
read abstract
ibidi Solutions:
|
 |





