Super-Resolution Microscopy (STED, SIM, (F)PALM, (d)STORM)
Applications:
Super-resolution microscopy enables the visualization of the smallest structures in living cells that cannot be resolved using standard widefield or confocal fluorescence microscopy. This technique provides a spatial 3D-resolution that is well below the diffraction limit. It creates new views on the structural organization of cells and the dynamics of biomolecular assemblies, that are close to a near-molecular resolution.
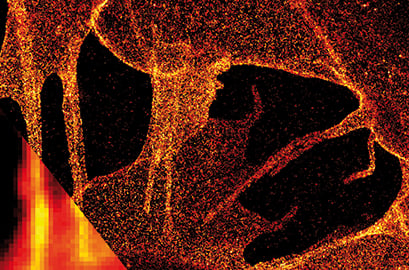
dSTORM image of plasma membrane glycans on the ibidi Polymer Coverslip. Membrane glycans of SK-N-MC neuroblastoma cells were stained through the metabolic incorporation of azido-sugar analogues followed by copper-catalyzed azide-alkyne cycloaddition (CuAAC). Inlet: comparison to widefield microscopy. Provided by Markus Sauer, Würzburg
Principle:
Resolution is described as a function to discriminate two dots from each other. It is dependent on the wavelength and the numerical aperture and is physically limited by Abbe's law.
When using widefield and confocal fluorescence microscopy, the diffraction barrier limits the maximal resolution to about 200 nm. Super-resolution microscopy breaks the diffraction barrier, enabling "nanoscopy" with substantially improved optical resolution of down to 5–20nm. This method uses the physical or chemical properties of adjacent fluorophores to resolve them from each other. For example, while one fluorophore's state is "on", the neighboring fluorophore's state is "off", which enables their differentiation.
Several super-resolution microscopy techniques were developed, each with its own advantages and disadvantages. There are deterministic and stochastic functional techniques. Common examples are:
- Stimulated emission depletion (STED)
- Saturated structured illumination microscopy (SSIM)
- REversible Saturable Optical Linear Fluorescence Transitions (RESOLFT)
- Photoactivated localization microscopy (PALM)
- Fluorescence photoactivation localization microscopy (FPALM)
- Stochastic optical reconstruction microscopy (d)STORM
Find more detailed information about the principle of super-resolution microscopy and its different approaches in the publications below.
L. Woythe, P. Madhikar, N. Feiner-Gracia, C. Storm, L. Albertazzi. A Single-Molecule View at Nanoparticle Targeting Selectivity: Correlating Ligand Functionality and Cell Receptor Density. ACS Nano, 2022, 10.1021/ACSNANO.1C08277
Read article
S.W. Hell. Far-field optical nanoscopy. Science, 2007, 10.1126/science.1137395
Read article
S.W. Hell. Microscopy and its focal switch. Nat Methods, 2009, 10.1038/nmeth.1291
Read article
B. Huang, H. Babcock, X. Zhuang. Breaking the diffraction barrier: super-resolution imaging of cells. Cell, 2010, 10.1016/j.cell.2010.12.002
Read article
S.J. Sahl, S.W. Hell, S. Jakobs. Fluorescence nanoscopy in cell biology. Nat Rev Mol Cell Biol, 2017, 10.1038/nrm.2017.71
Read article
ibidi Solutions:In general, we can recommend the ibidi Glass Coverslip bottom for super-resolution microscopy techniques. The ibidi Polymer Coverslip, however, cannot yet be guaranteed to work with every microscopic technique due to the large number of techniques available. Please note: dSTORM is possible with the ibidi Polymer Coverslip. If you would like to take advantage of the ibidi Polymer Coverslip bottom features and test them with your own super-resolution microscopy experiments, please check out our free sample! Then, give us feedback on how the ibidi Polymer Coverslip worked with your technique of choice. |
 |





