5 Versatile in Vitro Assays for Studying Neurological Conditions and Diseases
ibidi Blog | March 17, 2023 | Tina Freisinger, ibidi GmbH
Neurological conditions represent a significant health burden worldwide, with almost 1 in 6 people suffering from neurological disorders and diseases.
These disorders affect the whole nervous system, including the brain, spinal cord, and peripheral nervous system (PNS), often resulting in disability or death. Neurological conditions can be caused by many different factors, such as environmental/nutritional influences, genetic mutations, or trauma. Examples are neurodegenerative diseases like different forms of dementia (e.g., Alzheimer’s), Parkinson’s disease, and amyotrophic lateral sclerosis (ALS), but also stroke, brain cancer, infectious diseases (e.g., meningitis), and injuries.
Neuroscience research focuses on studying the function and organization of the central nervous system (CNS) and peripheral nervous system (PNS) to understand neurological processes and develop biomarkers and therapies for neurological diseases and conditions.
Cell-based models and assays play an important role in molecular and cellular neurobiology, as they allow the study of neural cell types under controlled conditions and their response to various stimuli. In vitro assays can also be used to investigate the effects of disease-related changes in neural function to test potential therapies.
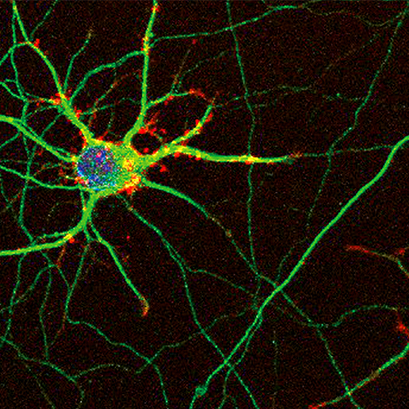
Confocal image of mouse hippocampal neurons grown on ibidi labware. The neurons were immunostained for bIII-tubulin (green), actin fibers (red), and nuclei (blue). Image courtesy: Ilaria Tonazzini, Institute of Nanosciences, CNR and NEST, Scuola Normale Superiore, Pisa, Italy
In this blog article, we introduce 5 in vitro cell-based assays to study neurological conditions and diseases and give application examples for each assay.
1. Fluorescence Microscopy Assays
Fluorescence microscopy assays such as Immunofluorescence and Fluorescence in situ hybridization (FISH) are used to visualize specific molecules or structures within cells or tissues using fluorescent tags or probes.
In cellular and molecular neurobiology, immunofluorescence assays (e.g., immunocytochemistry, immunohistochemistry, and in situ hybridization) can be used to gain important insights into the spatial organization of the brain and neural network. Furthermore, labeling proteins, DNA, and RNA allows visualization of their functional role within these networks and can reveal if processes are disrupted. Immunofluorescence is a versatile, straightforward method to visualize the location and interaction of cellular structures and components. Protocols and workflows are easy to establish in virtually every cell biology lab.
Multiplexing (the staining of multiple proteins simultaneously) even allows visualization of different molecules simultaneously, which can be very useful in deciphering the interaction of proteins, for example, in neural signaling processes.
Find out more about Immunofluorescence Assays and Applications.
Application Example 1: Immunohistochemistry and Fluorescence In Situ Hybridization (FISH) to Identify a Mutation Responsible for Familial ALS
ALS is a chronic and fatal neurodegenerative disease that results in the progressive loss of motor neurons. ALS leads to increasing muscle deterioration and weakness, eventually resulting in the loss of the ability to move, speak, eat, and breathe.
Most cases of ALS have no known cause (sporadic ALS), while 5 to 10% of the cases are genetic and can be linked to specific genes. Several mutations in the FUS gene were identified in patients with familial ALS.
Gadgil et al. used fluorescence in situ hybridization (FISH) and immunofluorescence to identify an ALS-linked FUS mutation (FUS P525L) which disrupts the localization of U7 small nuclear ribonucleoprotein (U7 snRNP). Furthermore, they could show that this mutant mis-colocalized, along with U7 snRNA, into the cytoplasm and affected the replication-dependent histone genes (Gadgil et al.,2021).
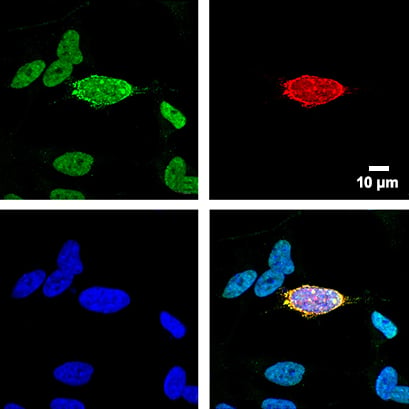
Fig.1 SHSY5Y FUS knockout cells were transfected with a plasmid containing the FUS gene harboring ALS-linked FUS P525L mutation. 48 hours post-transfection, the cells were co-stained for U7 snRNA and FUS. Green: FISH-staining of U7 snRNA. Red: FUS P525L. Blue: Nuclei (DAPI) in blue. Cells were stained and imaged in an ibidi Chamber with Coverslip Bottom.
Courtesy: by Ankur Gadgil, Laboratory of RNA Processing, Center for Advanced Technologies, Uniwersytetu Poznańskiego, Poznań, Poland.
2. Live Cell Imaging
Live Cell Imaging is a powerful tool to visualize cellular processes under physiological conditions in real time.
Neurodegenerative diseases like Alzheimer’s or multiple sclerosis (MS) are characterized by changes in dynamic cellular processes within the brain’s neural network. These altered processes can lead to dysfunctional protein transport and sorting as well as protein aggregation in the brain. Neural migration, chemotaxis, and angiogenesis are also examples of dynamic processes that play an important role in neural health and disease (Application Examples 2–5).
Live cell imaging allows for following these dynamic processes under physiological conditions in real-time (Mov. 1).
Find out more about Live Cell Imaging.
3. Migration and Chemotaxis Assays
Cell migration and chemotaxis are fundamental processes in the nervous system and play important roles in the pathophysiology of various neural conditions. For example, they are involved in neural development, neuroinflammation after injury, and during brain cancer progression.
Cell migration dynamics can be studied on a single-cell-level by tracking individual cells during live cell imaging (Application Example 2) or by measuring the change in a cell-covered area by introducing a cell-free gap into a cell population and observing gap closure over time (Application Example 3, workflow Fig. 2).
Chemotaxis is the directed movement of cells in response to chemical signals. In vitro chemotaxis assays allow for a qualitative and quantitative analysis of cell migration towards a specific molecule (Application Example 4, workflow Fig. 3).
Find out more about Wound Healing/Migration Assays and Chemotaxis Assays.
Application Example 2: Live Cell Imaging of Migrating Primary Schwann Cells
Schwann cells are glia cells of the PNS. They support and protect neurons and play an important role in transmitting nerve impulses but also in nerve development and regeneration. Therefore, they are of great interest in regenerative medicine to develop therapies that enhance the migratory potential of Schwann cells in the case of neural injury. Millesi et al. investigated primary Schwann cell migration using live cell imaging (Mov. 1). They also explored the regenerative effects of native spider silk fibers as a treatment option for long-distance peripheral nerve defects (Millesi et al., 2021).
Mov. 1 Live cell imaging of Schwann cells with trajectory lines to follow their migration pattern. Cells were cultivated on a µ-Slide 4 Well and imaged using an ibidi Stage Top Incubation System. Cell tracking and analysis was done with the Image J Manual Tracking plugin and the Chemotaxis and Migration Tool.
Courtesy: F. Millesi, Department for Plastic, Reconstructive and Aesthetic Surgery, Medical University Vienna, Austria.
Application Example 3: Effect of Growth Differentiation Factor 11 (GDF11) Treatment on Axon Regrowth and Cell Migration
Traumatic spinal cord injury (SCI) can be the result of an accident or a disease. The inflicted injury is irreversible and often leads to loss of motoric function and paralysis.
Therapeutic intervention focuses on inhibiting the neurodegenerative process which follows the acute injury of the spinal cord. Tsai et al. investigated whether GDF11 affects nerve generation after spinal cord injury. They performed wound healing experiments (workflow see Fig. 2) on spinal cord neuron-glial cultures in the presence and absence of GDF11. They observed significantly enhanced cell migration and neurite extension in the presence of GDF11 after 2 days (Tsai et al., 2022).
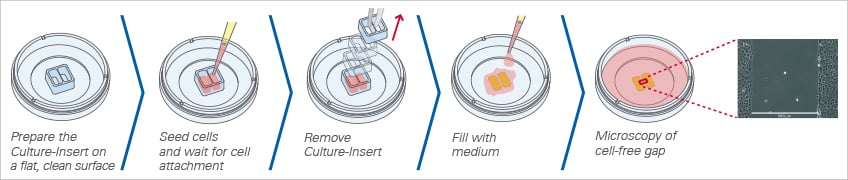
Fig. 2 Workflow for wound healing experiments with the Culture-Insert 2 Well.
Application Example 4: Chemotaxis Assays with Glioma Cells in Response to Chemokine C-C ligand 5
Glioblastoma is a fast-growing, highly-invasive malignant brain tumor with a poor prognosis (1.5 years median survival). There are a variety of treatments available (surgery, radiation, chemotherapy), but tumor reoccurrence is common due to resistant residual glioblastoma stem cells. The tumor microenvironment plays a crucial role in its development and progression. Glioma-associated macrophages (GAMs) are immune cells infiltrating in the tumor microenvironment and are implicated to promote tumor growth, angiogenesis, and invasion. GAMs have been shown as a source for C-C motif chemokine ligand 5 (CCL5), an abundant chemokine that plays a pro-inflammatory role in the cellular immune response. Its expression in GAMs further enhances tumor progression by triggering intracellular calcium signaling and inducing matrix metalloproteinases (MMPs).
Yu-Ju Wu et al. set up a 3D chemotaxis assay (workflow Fig. 3) to investigate the chemotactic invasion of glioma cells in the presence and absence of granulocyte-macrophage colony-stimulating factor (GM-CSF), which is present in the glioma microenvironment in vivo (Yu-Ju Wu et al., 2020).
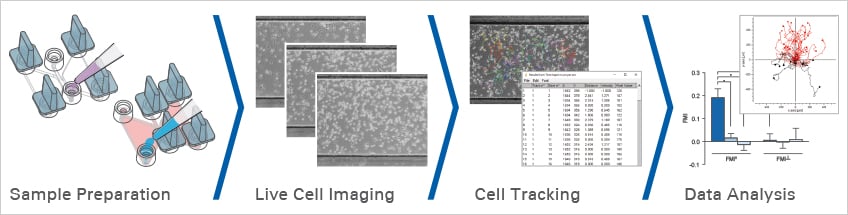
Fig. 3 Workflow for chemotaxis experiments with the µ-Slide Chemotaxis.
4. Tube Formation Assays
Blood vessel development (angiogenesis) is a crucial process in neural health and disease. Angiogenesis requires a cascade of cellular events, which is triggered by hypoxia.
After an ischemic stroke or traumatic brain injury, the resulting hypoxia triggers new blood vessel formation and therefore restores blood flow to the brain to ensure its supply of oxygen and nutrients.
The formation of new blood vessels can also have negative implications, as it is the case during (brain) cancer development. The hypoxic conditions in the tumor microenvironment induce blood vessel formation to ensure the nutrient supply of the tumor, thereby promoting its growth and spreading.
The tube formation assay (workflow Fig. 4) is a widely established in vitro method to study angiogenesis and to find angiogenesis-promoting or inhibiting compounds. In neurodegenerative research, tube formation assays can be used to develop therapies that promote vessel regeneration after acute brain injury (Application Example 5) or to characterize molecules that inhibit the formation of blood vessels that supply brain tumors with nutrients.
Find out more about Angiogenesis Assays and Applications.
Application Example 5: Tube Formation Assay to Study Vessel Regeneration After Traumatic Brain Injury
Traumatic brain injury (TBI) can affect cerebral blood vessels in several ways, leading to disruption of the blood brain barrier (BBB), increased intracranial pressure, cerebral vasospasm, and hemorrhage. These effects can contribute to the severity of brain damage and neurological deficits following TBI. FGF20, a member of the fibroblast growth factor family, has been shown to have a neuroprotective effect and therefore has been implicated in the treatment of Parkinson’s disease.
Guo et al. were interested to find out whether FGF20 also has a protective effect on the cerebral vasculature after TBI. Therefore, they used tube formation assays (workflow Fig. 4.) to investigate whether FGF20 can promote angiogenesis in immortalized human brain microvascular endothelial cells (hCMEC/D3) in vitro (Guo et al., 2021).
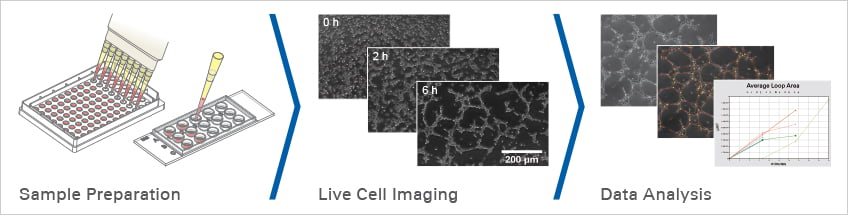
Fig. 4 Workflow for angiogenesis experiments with the µ-Slide 15 Well 3D.
5. Cell Culture Under Flow Assays
Cell culture under flow is an in vitro method to simulate the physiological conditions of blood/lymphatic vessels and to study the effects of mechanical forces (shear stress) created by the fluid flow on endothelial cells. This technique has been used in various fields of research, including neuroscience, to study neurovascular function and neuropathology.
Cell culture under flow allows researchers to investigate the effects of mechanical forces on endothelial brain vessel cells and the BBB in a controlled environment. By subjecting endothelial cells (ECs) to fluid flow, researchers can mimic the physiological conditions of the BBB and gain insights into the mechanisms underlying its function and dysfunction (Application Example 6).
Find out more about Cell Culture Under Flow Assays and Applications.
Application Example 6: Cell Culture Under Flow of hCMEC/D3 Cells
As with all ECs, human cerebral microvascular ECs are subjected to constant mechanical forces (shear stress) generated by the blood flow. In contrast to peripheral ECs, little is known about the cellular and molecular effects of shear stress on hCMECs.
In a recent study, Choublier et al. cultivated hCMEC/D3 cells under physiological laminar flow to study the effects of shear stress in vitro (workflow Fig. 5).
They used hCMEC/D3 cells as an in vitro model to study the BBB since it displays many key properties of the BBB, including tight junctions, transporters, and enzymes. They could show that, in contrast to peripheral endothelial cells that align in the direction of the flow, hCMEC/D3 cells align perpendicularly to the flow direction. Subsequent proteomic analysis revealed that laminar physiological shear stress induces quiescence of hCMEC/D3 cells, leading to protective and anti-inflammatory effects of ECs and, presumably, also the BBB (Choublier et al., 2022).
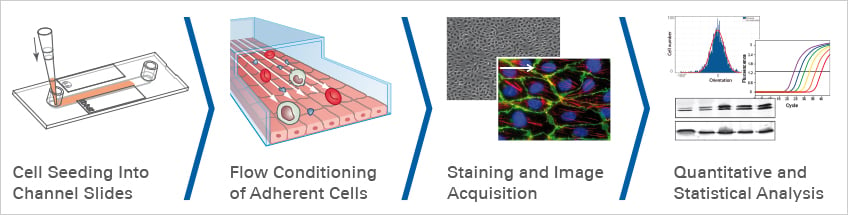
Fig. 5 Workflow for cell culture under flow experiments with ibidi Channel Slides and the ibidi Pump System.
For more information on neuroscience research, visit our Neuroscience Application Site.
References
Ehrenberg AJ, Morales DO, Piergies AMH, Li SH, Tejedor JS, Mladinov M, Mulder J, Grinberg LT. A manual multiplex immunofluorescence method for investigating neurodegenerative diseases. J Neurosci Methods. 2020 Jun 1;339:108708. doi: 10.1016/j.jneumeth.2020.108708.
Read article
Gadgil A, Walczak A, Stępień A, Mechtersheimer J, Nishimura AL, Shaw CE, Ruepp MD, Raczyńska KD. ALS-linked FUS mutants affect the localization of U7 snRNP and replication-dependent histone gene expression in human cells. Sci Rep. 2021 Jun 4;11(1):11868. doi: 10.1038/s41598-021-91453-3.
Read article
Bakota L, Brandt R. Live-cell imaging in the study of neurodegeneration. Int Rev Cell Mol Biol. 2009;276:49-103. doi: 10.1016/S1937-6448(09)76002-2.
Read article
Millesi F, Weiss T, Mann A, Haertinger M, Semmler L, Supper P, Pils D, Naghilou A, Radtke C. Defining the regenerative effects of native spider silk fibers on primary Schwann cells, sensory neurons, and nerve-associated fibroblasts. FASEB J. 2021 Feb;35(2):e21196. doi: 10.1096/fj.202001447R.
Read article
Datta D, Subburaju S, Kaye S, Baruah J, Choi YK, Nian Y, Khalili JS, Chung S, Elkhal A, Vasudevan A. Human forebrain endothelial cell therapy for psychiatric disorders. Mol Psychiatry. 2021 Sep;26(9):4864-4883. doi: 10.1038/s41380-020-0839-9.
Read article
Tsai MJ, Fay LY, Liou DY, Chen Y, Chen YT, Lee MJ, Tu TH, Huang WC, Cheng H. Multifaceted Benefits of GDF11 Treatment in Spinal Cord Injury: In Vitro and In Vivo Studies. Int J Mol Sci. 2022 Dec 27;24(1):421. doi: 10.3390/ijms24010421.
Read article
Yu-Ju Wu C, Chen CH, Lin CY, Feng LY, Lin YC, Wei KC, Huang CY, Fang JY, Chen PY. CCL5 of glioma-associated microglia/macrophages regulates glioma migration and invasion via calcium-dependent matrix metalloproteinase 2. Neuro Oncol. 2020 Feb 20;22(2):253-266. doi: 10.1093/neuonc/noz189.
Read article
Codrici E, Popescu ID, Tanase C, Enciu AM. Friends with Benefits: Chemokines, Glioblastoma-Associated Microglia/Macrophages, and Tumor Microenvironment. Int J Mol Sci. 2022 Feb 24;23(5):2509. doi: 10.3390/ijms23052509.
Read article
Zhu H, Zhang Y, Zhong Y, Ye Y, Hu X, Gu L, Xiong X. Inflammation-Mediated Angiogenesis in Ischemic Stroke. Front Cell Neurosci. 2021 Apr 21;15:652647. doi: 10.3389/fncel.2021.652647.
Read article
Abou Khouzam R, Brodaczewska K, Filipiak A, Zeinelabdin NA, Buart S, Szczylik C, Kieda C, Chouaib S. Tumor Hypoxia Regulates Immune Escape/Invasion: Influence on Angiogenesis and Potential Impact of Hypoxic Biomarkers on Cancer Therapies. Front Immunol. 2021 Jan 20;11:613114. doi: 10.3389/fimmu.2020.613114.
Read article
Gião T, Saavedra J, Vieira JR, Pinto MT, Arsequell G, Cardoso I. Neuroprotection in early stages of Alzheimer's disease is promoted by transthyretin angiogenic properties. Alzheimers Res Ther. 2021 Aug 24;13(1):143. doi: 10.1186/s13195-021-00883-8.
Read article
Guo R, Wang X, Fang Y, Chen X, Chen K, Huang W, Chen J, Hu J, Liang F, Du J, Dordoe C, Tian X, Lin L. rhFGF20 promotes angiogenesis and vascular repair following traumatic brain injury by regulating Wnt/β-catenin pathway. Biomed Pharmacother. 2021 Nov;143:112200. doi: 10.1016/j.biopha.2021.112200
Read article
Choublier N, Taghi M, Menet MC, Le Gall M, Bruce J, Chafey P, Guillonneau F, Moreau A, Denizot C, Parmentier Y, Nakib S, Borderie D, Bouzinba-Segard H, Couraud PO, Bourdoulous S, Declèves X. Exposure of human cerebral microvascular endothelial cells hCMEC/D3 to laminar shear stress induces vascular protective responses. Fluids Barriers CNS. 2022 Jun 3;19(1):41. doi: 10.1186/s12987-022-00344-w.
Read article
 (0)
(0)
 (0)
(0)





